
Photochemistry_of_Organic
.pdf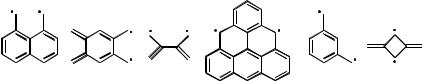
226 |
Photochemical Reaction Mechanisms and Reaction Intermediates |
2.Identify the symmetry point groups of formaldehyde [C2v], ammonia [C3v], phenol [Cs], gyloxal [C2h] and allene [D2h].
3.Verify Equation 4.31 for a configuration of six electrons in three orbitals by considering all electronic interactions.
4.Predict the ground-state multiplicity of the conjugated biradicals shown in Scheme 5.25. [T, S, S, T, T, T]519
Scheme 5.25 Non-Kekule biradicals
5.Calculate the energy gap between the lowest excited singlet and triplet configurations of ethene, E(1x1 ! 1) – E(3x1 ! 1) ¼ 2K1–1, using r12 ¼ 134 pm and the PPP SCF parameters given below Equation 4.29 [5.407 eV].
6.Calculate the concentration required to quench 99% of singlet excited naphthalene, given that the lifetime of the naphthalene singlet state is 100 ns in the absence of
dioxygen and N,N-dimethylaniline and that the rate constant of diffusion kdiff is
5 109 M 1 s 1. [0.2 M]
7.Explain why the oscillator strengths of charge-transfer transitions in EDA complexes are low and why the absorption maximum of the charge-transfer band of
tetracyanoethylene complexes with methylated benzenes is red shifted upon increasing methyl substitution (from n~max ¼ 2.6 mm 1 for benzene to n~max ¼ 1.9 mm 1 for hexamethylbenzene).343
8.Rationalize the singlet–triplet energy gaps of the carbenes listed in Table 5.1.

6
Chemistry of Excited Molecules
6.1 Alkenes and Alkynes
The absorption spectra of unsaturated hydrocarbons are dominated by p,p transitions, which were discussed in Sections 4.5 and 4.7. Some representative spectra are shown on a logarithmic scale in Figure 6.1. The first strong absorption band, log(«/[M 1 cm 1]) 4, of unsaturated hydrocarbons usually corresponds to the HOMO–LUMO transition that is well described by H€uckel theory. However, two types of weak transitions may in fact appear at lower energies, which can easily pass unnoticed upon cursory inspection of the corresponding absorption spectra, but may determine the photoreactivity. The first are the parity-forbidden transitions (Section 4.7), log(«/[M 1 cm 1]) 2.5, which occur in all alternant hydrocarbons. They represent the lowest singlet state S1 in important chromophores such as benzene, naphthalene and their derivatives, as exemplified by the spectra of styrene and phenylacetylene (Figure 6.1, bottom). The second are the symmetry-forbidden 1Ag bands, log(«/[M 1 cm 1]) 1 in linear polyenes and their phenyl derivatives (Section 4.7). In terms of MO theory, the lowest 1Ag state is described by a doubly excited configuration with two electrons promoted from the HOMO to the LUMO. Even when the 1Ag state is not the lowest excited singlet state, it may nevertheless be important for photoreactivity, as in polyenes, because the energy of the 1Ag state is lowered along reaction coordinates of double bond twisting (Section 5.5). Donor and acceptor substituents, on the other hand, affect mostly the strong HOMO–LUMO transition, shifting it to lower energies.
Twisting of essential single bonds in the ground state due to steric interactions is associated with a hypsochromic shift of the HOMO–LUMO absorption maximum. This effect is seen in the absorption spectra of (E)- and (Z)-stilbene (Figure 6.1, bottom). On the other hand, the red edge of the (Z)-stilbene absorption extends to lower energies; the nonvertical transition to the relaxed excited state requires less energy, because twisting destabilizes the ground state. The p-systems of 1,3-dienes (1,10-bicyclohexene, Figure 6.1,
Photochemistry of Organic Compounds: From Concepts to Practice Petr Klán and Jakob Wirz © 2009 P. Klán and J. Wirz. ISBN: 978-1-405-19088-6

228 |
Chemistry of Excited Molecules |
Publisher's Note:
Permission to reproduce this image online was not granted by the copyright holder. Readers are kindly requested to refer to the printed version of this chapter.
Figure 6.1 Absorption spectra of prototype alkenes and alkynes. Top: 1,10-bicyclohexene280
(—), provitamin D520 (7-dehydrocholesterol; —), cycloocta-1,3,5-triene520 (– – –), tetramethylethylene280 (- - -), and 2,3-dimethylbut-2-enal280 (....). Bottom: (E)-stilbene521 (—), (Z)-stilbene521 (....), phenylacetylene522 (—), styrene280 (- - -)
top) and a,b-unsaturated ketones or aldehydes (2,3-dimethylbut-2-enal) are isolectronic and exhibit similar p,p bands. However, ketones and aldehydes exhibit an additional, weak n,p transition at longer wavelengths.
The singlet lifetimes of unsaturated hydrocarbons vary from picoseconds (stilbenes) to nearly 1 ms (pyrene). Radiative lifetimes tr ¼ 1/kf range from about 1 ns for compounds with a strong S0 ! S1 absorption band to many microseconds with compounds having a forbidden S0 ! S1 absorption band. The photophysical properties of some prototype unsaturated hydrocarbons are listed in Table 8.6. Radiationless decay associated with E–Z (or trans–cis) isomerization is usually very fast in polyenes (see below). Intersystem crossing is generally slow – too slow to compete efficiently with these deactivation processes, because spin–orbit coupling is weak and the singlet–triplet energy gap is large in alternant hydrocarbons. Therefore, unsaturated hydrocarbons that do not undergo E–Z isomerization – due to steric constraints or in extended systems – have high fluorescence quantum yields.
Alkenes and Alkynes |
229 |
Table 6.1 lists the principal photoreactions of alkenes and alkynes that we describe in this Section. It is structured according to the types of photoreactions that are characteristic for an initial reacting chromophore. Many practical examples and special topics will expand on this general information and a number of references lead the reader to review articles or the original reports.
Most molecules containing a C¼C bond are predestined to undergo photoinduced geometric E–Z isomerization (Table 6.1, entry 1), unless the double bond is embedded in a small ring or the molecule is constrained by a rigid environment. Since the E- and Z-isomers have different absorption properties, monochromatic light may be used to enrich the one that absorbs less at the excitation wavelength. The photoisomerization mechanism of singlet and triplet excited alkenes differs (Section 5.5); however, the reactant multiplicity has only a minor impact on the overall reaction. Direct irradiation of monoalkenes is difficult because they absorb at short wavelengths; therefore, photosensitization is a practical technique to carry out the reaction. When the double bond is conjugated to an auxochrome, such as a carbonyl group, the triplet state can be readily formed via intersystem crossing.
E–Z isomerization, including chemically nonproductive isomerization about terminal double bonds, will tend to lower the quantum yields of competing photoreactions such as electrocyclization or sigmatropic shift (entries 2 and 3) of dienes, respectively. The cyclization products absorb at shorter wavelengths than the reactant diene, so that high chemical yields of cyclobutenes may nevertheless be achieved by suppressing secondary photoreactions of the photoproduct such as cycloreversion (reverse opening) with appropriate cut-off filters. When two double bonds are connected through a methylene bridge, the di- p-methane rearrangement (entry 4) can be the principle reaction for the same reasons.
Excited alkenes and alkynes are highly reactive towards nucleophiles, acids and electron donors. Nucleophilic addition and photoreduction (entries 5 and 8) predominate with alkenes carrying electron-withdrawing substituents. Some electron-rich alkenes or alkynes readily undergo photoprotonation (entries 6 and 7).
Bimolecular photocycloaddition (entry 9) of an excited alkene to a ground-state alkene is clearly influenced by alkene concentration. However, close proximity of the reactants can also be promoted by complexation or by constrained environments.
6.1.1Alkenes: E–Z Isomerization
 R hν R R
R hν R R
R hν
Recommended review articles.500,523–533
Selected theoretical and computational photochemistry references.16,534–552
E–Z (or trans–cis) isomerization (Section 5.5) of alkenes, involving a 180 rotation
about a C¼C bond, is known to be initiated both thermally and photochemically.524– 526,530,553 After equilibrating, the thermal process produces a mixture of the two isomers
in a ratio that reflects their relative thermodynamic stabilities. In contrast, photoisomerization of an alkene (Scheme 6.1) is governed by the ratio of the E ! Z and
230 |
|
|
Chemistry of Excited Molecules |
|
|||
Table 6.1 Photoprocesses involving excited alkenes and alkynes |
|
||||||
|
|
|
|
|
|
|
|
Entry Starting materiala |
Product(s) |
Mechanism |
Section |
||||
|
|
|
|
|
|
|
|
1 |
|
R * |
R |
|
R |
E–Z isomerization |
6.1.1 |
|
|
|
|||||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R
2
3
4
5
6
7
8
9
*
 *
*
R
R R  *
*
*
+ Nu
*
+ H
*
R  + H3O
+ H3O
*
+ [e ]
]
 *
*
+
Electrocyclization 6.1.2
Sigmatropic shift |
6.1.2 |
R
R R
Di-p-methane 6.1.3 rearrangement
|
Nu |
Photoinduced |
6.1.4 |
|
nucleophile |
|
|
|
|
|
|
|
|
addition |
|
|
H |
Photoinduced proton |
6.1.4 |
|
addition |
|
|
|
|
|
|
HO |
O |
|
|
R |
R |
Photoinduced proton |
6.1.4 |
addition |
|
||
|
|
Photoreduction |
6.1.4 |
Photocycloaddition |
6.1.5; 6.1.6 |
aNu ¼ nucleophile; [H] ¼ hydrogen atom donor; [e ] ¼ electron donor.
Alkenes and Alkynes |
231 |
Z ! E isomerization quantum yields (FE–Z and FZ–E, respectively) and the molar absorption coefficients « of the corresponding isomers. The resulting isomer ratio is reached when the rates of formation and disappearance for each isomer become equal (photostationary state; PSS). A large excess of the thermodynamically less stable Z-isomer can be produced when favourable absorption properties (higher « values) of the other E-isomer at certain wavelengths allow it (Figure 6.1, bottom). Other competing photoprocesses, such as radiation or radiationless decays (Section 2.1), sigmatropic shifts (Section 6.1.2), carbene formation, [2 þ 2] cycloadditions (Section 6.1.5) or electrocyclization reactions (Section 6.1.2), can also compete with this reaction.
|
|
|
E-Z |
|
|
|
|
|
|
|
|
|||
|
|
isomerization |
|
|
|
|
|
|
|
|
||||
R1 |
|
hν |
R1 R2 |
|
|
|
λ |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R2 |
|
hν |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
(Z)-isomer |
|
|
|
λ |
|
||||||
(E)-isomer |
|
|
|
|
|
|
|
|
|
|||||
Scheme 6.1
E–Z isomerization of alkenes initiated by direct irradiation usually proceeds from the excited singlet state. Because of the existence of two or more close-lying excited singlet
states, including the Rydberg [p,R(3s)] states,554 slow mutual interconversion between the excited states is probably involved.555,556 The presence of a triplet sensitizer is required to
carry out the isomerization from a low-lying excited triplet state (p,p ). In such a case, the photostationary alkene concentrations depend on the ratios of the rate constants of energy
transfer to both isomers, the magnitudes of which are associated with the relative sensitizer triplet energies (Section 2.2.2).525,553
Direct Irradiation
Absorption spectra of simple aliphatic alkenes in solutions, generally having their maxima (lmax) below 200 nm, imply that direct irradiation must be conducted with vacuum UV sources (Section 3.1). However, a medium-pressure mercury lamp with a quartz filter can be applied for highly substituted alkenes, possessing lmax over 200 nm. Direct irradiation of (Z)-oct-2-ene (36, R ¼ C4H9) at 185 nm leads to a nonselective excitation and consequently the E–Z isomerization and the 1,3-hydrogen migration products 37 and 38
via carbene intermediates (Scheme 6.2). This rearrangement possibly does not involve the 1p,p state but occurs via a Rydberg excited state and carbene intermediates.523,556
E–Z isomerization is, however, exclusively observed upon irradiation above 200 nm.556
Rearrangement and decomposition are the major reactions observed when small-ring cycloalkenes are photolysed.523,524,530E–Z photoisomerization becomes an important
process in mediumand large-ring cycloalkenes, although it is usually accompanied by side-reactions. Although isomerization in cyclopentene is still not geometrically feasible, (Z)-cyclohexene (39) gives the highly strained and unstable E-isomer by both direct (Scheme 6.3) and sensitized irradiation.557 This short-lived intermediate undergoes
232 |
Chemistry of Excited Molecules |
|
||
|
|
R hν |
R |
|
|
36 |
hν |
|
|
|
|
|
|
|
|
hν |
|
hν |
|
R |
|
R |
R |
R |
|
|
+ |
|
|
37 |
|
|
|
38 |
Scheme 6.2
non-stereospecific [2 þ 2] addition or radical side-reactions in aprotic non-nucleophilic solvents (Section 6.1.5). In contrast, cycloheptene shows little tendency to undergo photodimerization and (Z)-cyclooctene is the smallest cycloalkene producing the corresponding isolable E-isomer upon irradiation at 185 nm.558
hν
39
Scheme 6.3
Arylalkenes, such as stilbene derivatives, are important model compounds for the study of the E–Z photoisomerization.529,559,560 The compounds absorb significantly over
250 nm, therefore direct irradiation is technically simple. Photolysis of unsubstituted stilbene in aliphatic hydrocarbons at 313 nm affords a photostationary state (PSS) consisting of 93% of (Z)-stilbene and 7% of (E)-stilbene (Scheme 6.4).112 In addition, a
photoinduced 6p-electrocyclic (Section 6.1.2) formation of dihydrophenanthrene with a quantum yield of F 0.10 competes with (Z)-stilbene isomerization (FZ–E ¼ 0.35).487,561
|
|
|
Ph |
|
hν |
|
Ph |
|
|
Ph hν |
|
H |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
Ph |
|
hν |
|
|
|
|
|
|
∆ |
|
|||
|
|
|
|
|
|
|
|
|
|
|||||
PSS: |
7% |
|
|
|
|
|
93% |
|
|
|
|
|||
ε 313 /M-1 cm-1: |
19420 |
|
|
|
|
2510 |
|
|
|
|
||||
Scheme 6.4
The multiplicity of the excited state that is responsible for photoisomerization may vary with the nature of the stilbene phenyl ring substituents (Table 6.2). When the coupling
|
|
Alkenes and Alkynes |
|
233 |
|
Table 6.2 |
Photolysis of the stilbene derivatives Ph HC ¼ CH C6H4X529 |
|
|||
X |
FE–Za |
FZ–Ea |
Mechanismb |
[E]:[Z] (PSS) |
|
|
|
|
|
|
|
H |
0.52 |
0.35 |
S1 |
þ T1 |
93:7 |
4-Cl |
0.60 |
0.42 |
S1 |
91:9 |
|
4-Br |
0.52 |
0.35 |
S1 |
þ T1 |
88:12 |
aFE–Z and FZ–E are the quantum yields for E ! Z and Z ! E isomerization, respectively. bIsomerization from the singlet state (S1) or/and the triplet state (T1).
between the singlet and triplet manifolds increases, for example due to the heavy-atom effect (Section 4.8) of a bromine atom, a less selective triplet isomerization pathway becomes competitive.529
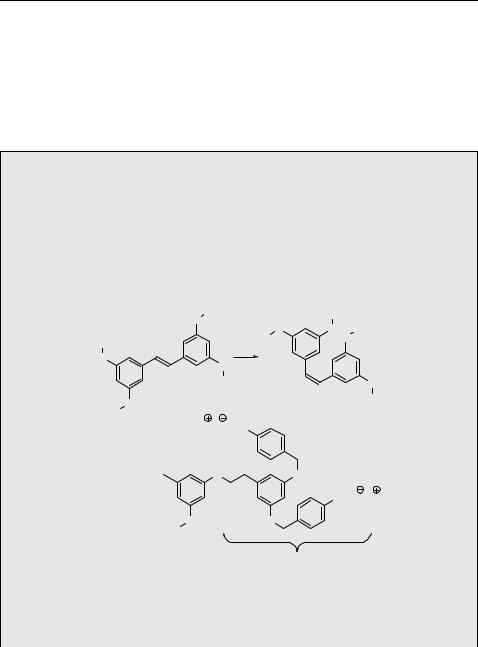
Case Study 6.1: Supramolecular chemistry – photoresponsive stilbene dendrimers
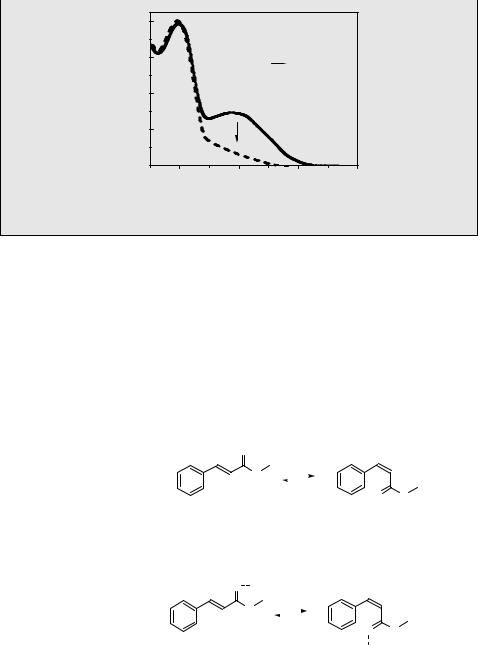
The water-soluble (E)-stilbene dendrimer 40 was found to undergo an unusual one-way E–Z isomerization to give the thermodynamically less stable (Z)-40 nearly exclusively at the photostationary state upon irradiation in water (Scheme 6.5).562 Monitoring the absorbance at 330 nm in the course of irradiation showed that the system reaches a photostationary state (Figure 6.2).
|
|
R |
|
R |
|
|
|
O |
R O |
O O R |
|
R |
|
hν |
|
||
|
|
|
|
||
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
O |
O |
(E)-40 |
|
|
(Z)-40 |
R |
|
|
|
|||
R |
|
|
|
|
|
|
|
K OOC |
|
|
|
|
R = |
O |
|
O |
|
|
|
|
|
COO K |
|
|
R' |
O |
O |
|
|
R'
Scheme 6.5
Experimental details.562 A KOH aqueous solution (2 10 3 M) of 40 was irradiated at 330 nm (Figure 3.9) at 20 C and product formation was followed by UV spectroscopy.
234 |
Chemistry of Excited Molecules |
|
1.6 |
|
|
|
|
1.2 |
(E)-40 |
(Z)-40 |
|
absorbance |
|
|
||
0.8 |
|
|
|
|
0.4 |
|
|
|
|
|
|
|
|
|
|
0.0 |
|
|
|
|
280 |
320 |
360 |
400 |
wavelength / nm
562
Figure 6.2 Change in absorption spectra during the photolysis of (E)-40
Lewis acid (BF3 or EtAlCl2) complexes of a,b-unsaturated esters can shift the photoequilibrium (PSS) toward the thermodynamically less stable Z-isomer even more and may inhibit other competing unimolecular photochemical processes.563 Such enhanced isomerization results are explained by selective excitation of the ground-state Lewis acid–ester carbonyl complex, which exhibits a red shift in the long-wavelength p,p absorption band (lmax) and higher molar absorption coefficients («313) (Scheme 6.6).
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
hν |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
hν |
|
O |
O |
||
|
|
|
|
|
|
|
|
|
|
||
PSS: |
54% |
|
|
|
|
|
|
|
46% |
|
|
λmax / nm (ε 313 /M-1 cm-1): |
277 (2.60) |
|
|
|
|
|
|
|
269 (2.70) |
|
|
|
O |
BF3 |
|
|
|
|
|
|
|
||
|
O |
|
|
hν |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
hν |
|
O |
O |
|||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
BF3 |
|
PSS: |
12% |
|
|
|
|
|
|
|
88% |
|
|
λmax / nm (ε 313 /M-1 cm-1): |
313 (4.52) |
|
|
|
|
|
|
|
303 (4.06) |
|
|
Scheme 6.6
Alkenes and Alkynes |
235 |
Extended conjugation shifts the strong p,p absorption bands of alkenes to higher wavelengths (Figure 6.1). An explanation of their photoisomerization is based on the idea of ground-state conformational control of photoreactivity, termed the NEER principle (non-equilibration of excited rotamers), according to which the excited species of the various ground-state rotamers (Section 5.5) will not equilibrate during their short singlet excited-state lifetime.564 The increased bond order between the unsaturated units of polyenes inhibits s-cis $ s-trans interconversion in the excited state. As a result, the photoproduct concentrations reflect the composition of the ground-state conformational equilibrium.
Three different photoisomerization mechanisms of singlet excited polyenes have
been proposed: (1) one-bond twist, a typical process observed in fluid solutions but also in glassy media,525,553,565 (2) the bicycle-pedal mechanism involving simulta-
neous rotation about two original double bonds, assumed to occur in a constraining environment,566–568 and (3) a volume-conserving two-bond hula twist 531,569,570
(Scheme 6.7). In some cases, the existence of the last mechanism has been ruled
out.567,571
H |
|
|
H |
|
H |
hν |
Ph |
H |
|
Ph |
|
|||
Ph |
one-bond |
|
H |
|
H |
|
H |
||
H |
twist |
|
Ph |
|
|
|
|||
(Z), (Z) |
|
(Z), (E) |
||
H |
|
Ph |
H |
|
H |
|
|||
hν |
|
|
||
Ph |
|
H |
||
Ph |
|
H |
||
bicycle-pedal |
|
|||
H |
|
|
||
H |
mechanism |
|
H Ph |
|
|
|
|||
(Z), (Z) |
|
(E), (E) |
||
H |
|
Ph |
H |
|
|
|
|||
H |
hν |
|
Ph |
|
|
|
|||
Ph |
|
H |
H |
|
Ph |
hula twist |
|||
|
|
|||
H |
|
H |
||
H |
|
|
||
|
(E), (Z) |
|||
(Z), (Z) |
|
|||
Scheme 6.7
Photochemistry of previtamin D (41; see also Special Topic 6.4) is an example of E–Z isomerization in trienes. Thermal (D) interconversion of its conformers 41a,b is suppressed in a cold, rigid matrix of a solidified medium (92 K) (Scheme 6.8). Upon
irradiation, 41a isomerizes to the thermodynamically more stable tachysterol conformer 42a, whereas 42b is formed exclusively from the conformer 41a.572,573
