
Photochemistry_of_Organic
.pdf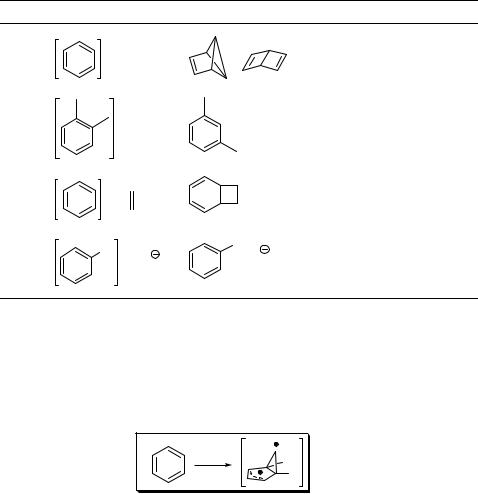
276 |
Chemistry of Excited Molecules |
Table 6.4 Photoprocesses involving excited aromatic compounds.
Entry Starting materiala Product(s) Mechanism Section
*
1 Photorearrangement 6.2.1
*
2 Phototransposition 6.2.1
|
* |
|
|
|
|
|
3 |
+ |
|
|
Photocycloaddition |
6.2.2 |
|
|
X |
* |
Y |
|
|
|
4 |
+ X |
Photosubstitution |
6.2.3 |
|||
|
+ Y |
|||||
|
|
|
|
|
aY ¼ nucleophile.
conditions, are responsible for the great diversity of reaction mechanisms and formation of specific products.
6.2.1 Aromatic Hydrocarbons and Heterocycles: Photorearrangement
and Phototransposition
Recommended review articles.784–788
Selected theoretical and computational photochemistry references.16,534,535,789–794
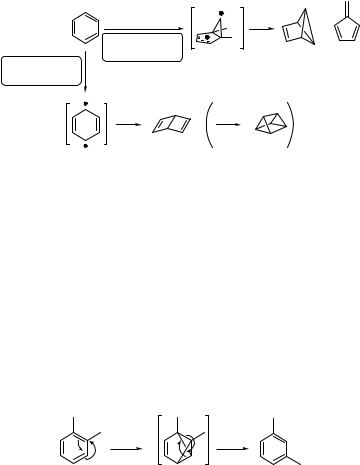
Benzene, the archetypical aromatic compound, possesses three absorption bands, at l ¼ 254 (S1), 203 (S2) and 180 nm (S3).785,788 Photolysis of benzene in the gas phase at 254 nm into the excited singlet state S1 (1B2u state; ES ¼ 459 kJ mol 1)157 produces two
non-aromatic highly strained products, benzvalene (168) (the limiting concentration attainable by irradiation of liquid benzene at higher temperature is only 0.05%) and fulvene (169), via a biradical intermediate 170 (prefulvene) with low quantum yields (F ¼ 0.01–0.03) (Scheme 6.74).795 The highly energetic non-aromatic Dewar benzene (171), along with 168 and 169, is obtained when benzene is irradiated with wavelengths at l < 200 nm. It has been shown that the S2 (1B1u ) state and the cyclohexa-2,5-dien-1,4-diyl
Aromatic Compounds |
|
277 |
|
254 nm |
H |
|
+ |
|
|
||
S1 state |
H |
|
|
isomerization |
170 |
168 |
169 |
S2 state
~200 nm
isomerization
hν
[2+2]
172 |
171 |
173 |
Scheme 6.74
biradical intermediate 172 are precursors of this product.796 Dewar benzene may further undergo [2 þ 2] intramolecular photocycloaddition (Section 6.1.5) to form prismane (173). Although intersystem crossing in benzene is relatively efficient (F ¼ 0.23 in hexane solution), the excited triplet state T1 (ET ¼ 353 kJ mol 1)157 is usually not involved in the photoisomerization.
Photochemical transposition (ring isomerization) of the carbon atoms in benzene is known to involve a benzvalene intermediate.784,785 Gas-phase photolysis of o-xylene (174)
at 254 nm, for example, leads to the benzvalene 175 and subsequently to m-xylene (176) at low conversion (<10%; Scheme 6.75).797 A para-isomer is formed upon prolonged irradiation.
|
254 nm |
|
|
low |
|
|
conversion |
|
174 |
175 |
176 |
Scheme 6.75
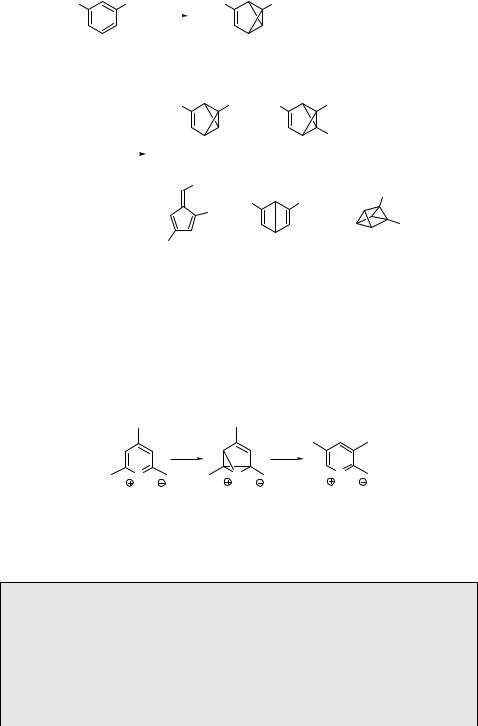
Isolation of reactive non-aromatic intermediates can be less problematic when the starting arenes are substituted with bulky substituents. Their stabilization is achieved especially by impeding the re-aromatization process through steric interactions. For example, 1,3,5-tri-tert-butylbenzene (177) produces 1,2,4-tri-tert-butylbenzvalene (178) as the sole product with F ¼ 0.12 upon photolysis at 254 nm (Scheme 6.76).798 Rearomatization and other processes become important after exhaustive irradiation, producing benzvalenes (179 and 180), fulvene (181), Dewar benzene (182) and prismane (183). The prismane derivative is obtained as the major product in 65% chemical yield at the photostationary state (PPS) (Section 6.1.1) because it does not absorb significantly at 254 nm. The S2 excited state is not available as a precursor to Dewar benzene (and its photocycloaddition product prismane) at this irradiation wavelength. It has therefore been suggested that the T1 excited state may be involved.785
278 |
|
|
|
|
Chemistry of Excited Molecules |
|
||||||||
t-Bu |
|
|
t-Bu 254 nm |
t-Bu |
|
t-Bu |
|
|
|
|||||
|
|
|
|
|
low |
|
|
|
|
|
|
|
|
|
|
|
|
|
conversion |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|||||||
|
t |
|
-Bu |
|
|
t-Bu |
|
|
|
|||||
|
177 |
|
|
|
|
|
178 |
|
|
|
|
|||
|
|
|
|
|
t-Bu |
|
|
t-Bu t-Bu |
t-Bu |
|
||||
|
|
|
|
|
|
|
|
|||||||
254 nm |
|
|
|
|
+ |
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
t-Bu |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
t |
-Bu |
|
|
|
180 |
|
|
|
photostationary |
|
|
|
|
|
||||||||
|
179 |
|
|
|
|
|
||||||||
|
|
|
state |
t-Bu |
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
t-Bu |
||
|
|
|
|
|
|
|
|
|
t-Bu |
t-Bu |
||||
|
|
|
|
|
|
|
|
|
|
|||||
|
+ |
|
|
|
t-Bu + |
+ t-Bu |
|
t-Bu |
||||||
|
|
|
|
|||||||||||
|
|
|
|
t-Bu |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
t-Bu |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
181 |
|
|
182 |
|
|
183 |
|||||||
Scheme 6.76
These photorearrangements are known to occur not only in substituted benzenes but also in substituted naphthalenes,785 pyridines,799 pyrylium cations,787 and pyridinium salts.786 For example, irradiation of 2,4,6-trimethylpyrylium perchlorate (184) results in the formation of the oxabenzvalene cation 185, which rearranges to the 2,3,5-trimethyl phototransposition isomer 186 (Scheme 6.77).800
|
hν |
|
O |
O |
O |
ClO4 |
ClO4 |
ClO4 |
184 |
185 |
186 |
Scheme 6.77
Case Study 6.13: Organic synthesis – photo-ring contraction
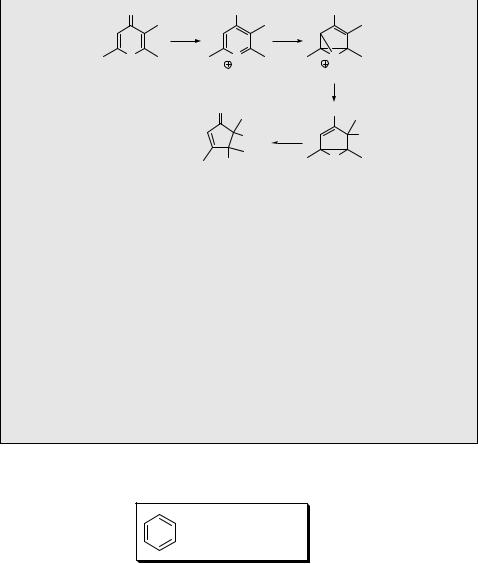
4-Hydroxypyrylium salts, produced in situ by protonation of 4-pyranones in aqueous H2SO4, undergo phototransposition and photo-ring contraction in high chemical yields.787 For example, photolysis of the 2,3-dimethyl-4-hydroxypyrylium cation 187 in 50% sulfuric acid was suggested to form the oxabenzvalene cation 188, which is trapped by water as a nucleophile, and the adduct 189 is subsequently hydrolysed to give the cyclopenten-2-one 190 (Scheme 6.78).801 At high H2SO4 concentrations,
Aromatic Compounds |
279 |
|
O |
OH |
OH |
50% |
hν |
|
H2SO4 |
|
|
O |
O |
O |
|
187 |
188 |
|
|
H2O |
|
O |
OH |
|
OH |
OH |
|
OH |
O |
|
|
|
|
190 |
189 |
Scheme 6.78
where the activity of nucleophilic water is low, the oxabenzvalene cation yields phototransposition products only.
Experimental details.801 4-Hydroxypyrylium cation (187) was prepared by dissolving the corresponding 4-pyrone (1.74 mmol) in 50% aqueous H2SO4 (8 ml). The acid solution in a quartz tube, placed in a quartz Dewar flask, was irradiated with six low-pressure Hg lamps (8 W; l ¼ 254 nm) arranged in a circular array around the Dewar flask (Figure 3.10). The sample temperature was maintained at 0 C during the irradiation by passing a stream of nitrogen through a heat exchanger coil immersed in a dry-ice–acetone bath and then through the quartz Dewar flask holding the sample. After irradiation, the acid solution was immediately neutralized and washed using diethyl ether. The aqueous layer was concentrated to dryness and a white solid was recrystallized from diethyl ether to give the product 190 in 63% chemical yield.
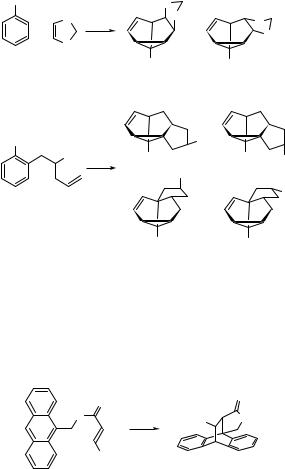
6.2.2Aromatic Hydrocarbons and Heterocycles: Photocycloaddition
+

 adducts
adducts
Recommended review articles.602,802–813
Selected theoretical and computational photochemistry references.814,815
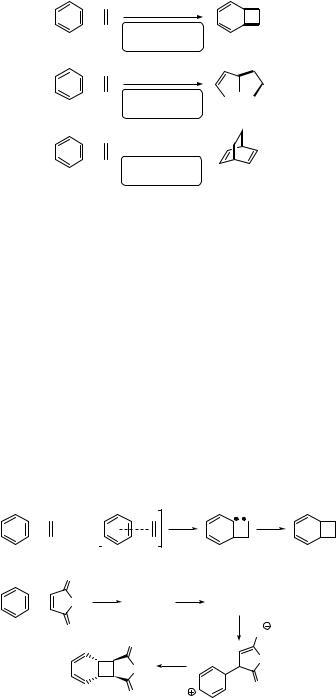
Benzene in the S1 (p,p ) excited state is no longer aromatic and is capable of undergoing various chemical reactions not observed in the ground state. Three basic types of
photocycloaddition reaction of an alkene to the excited benzene can be described:802,804,809,816 (a) bicyclo[4.2.0]octa-2,4-dienes (e.g. 191) are formed by ortho-
photocycloaddition (1,2- or [2 þ 2] photocycloaddition), (b) tricyclo[3.3.0.02.8]oct-3- enes (e.g. 192) are obtained by meta-photocycloaddition (1,3- or [3 þ 2]
Aromatic Compounds |
281 |
Ortho-Photocycloaddition
This reaction pathway is usually favoured when an aromatic moiety and an alkene
bear electron-withdrawing and electron-donating substituents, respectively (or vice versa).804,809 This addition involves a charge transfer and the course of the reaction is
sensitive to the solvent polarity. Such a mechanism may resemble that of [2 þ 2] photocycloaddition of alkenes to a,b-unsaturated carbonyl compounds (Section 6.3.2). Scheme 6.81 shows examples of two intermolecular processes and one intramolecular [2 þ 2] photocycloaddition reaction: (a) crotononitrile (196) is added to anisole (197) to yield several stereoisomers of 198 in 38% chemical yield and with high regioselectivity, which is linked to bond polarization in the exciplex;818 (b) hexafluorobenzene (199) reacts with 1-ethynylbenzene (200) to form the bicyclo[4.2.0]octa-2,4,7-triene 201 in 86% yield;819 and (c) irradiation of 202 in methanol leads to the single photoproduct 203. 820
(a) |
|
|
NC |
|
|
OMe |
|
MeO |
|
|
|
|
|
CN |
|
|
|
|
|
|
|
|
|
|
+ |
hν |
|
|
|
|
|
|
|
|
|
|
197 |
196 |
198 |
|
|
(b) |
F |
Ph |
F |
F |
Ph |
F |
F |
F |
|||
|
|
hν |
|
|
|
|
|
+ |
|
|
|
F |
F |
t-C4H9 |
F |
F |
t-C4H9 |
|
F |
F |
|
|
|
|
|
|
|
||
|
199 |
200 |
201 |
|
|
(c) |
|
O |
Ph |
|
O |
|
|
|
N |
|
|
|
|
Ph |
|
|
|
|
N |
hν |
|
|
|
|
|
Ph |
|
|
|
|
202 |
|
203 Ph |
||
Scheme 6.81
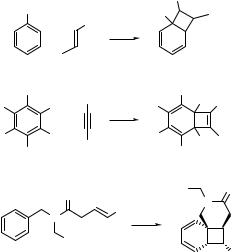
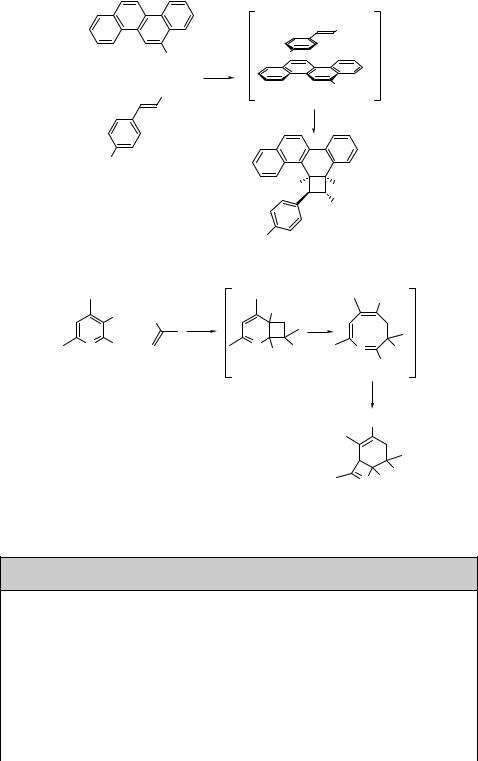
Polycyclic aromatic hydrocarbons are also known to undergo ortho-photocycloaddi- tions.805 For example, the reaction of the chrysene derivative 204 with the electrondeficient methyl cinnamate 205 affords the adduct 206 as the major product (Scheme 6.82).821 The high stereoselectivity observed has been explained by the formation of an electronically favourable sandwich-type singlet exciplex 207.
ortho-Photocycloaddition has also been observed in aromatic heterocyclic compounds, such as substituted pyridines. For example, irradiation of 2-methoxy-4,6-dimethylnico- tinonitrile (208) in the presence of methacrylonitrile (209) in benzene leads to the addition product 210 that thermally converts to the 3,4-dihydroazocine 211, which then undergoes electrocyclization in a second photochemical step to give 212 in 45% chemical yield (Scheme 6.83).822
282 |
|
|
Chemistry of Excited Molecules |
|
|
||
|
|
|
|
1 |
|
CO2Me * |
|
|
|
|
|
|
|
||
|
|
204 |
CN |
MeO |
|
|
|
|
|
+ |
|
hν |
|
|
|
|
|
|
|
|
CN |
|
|
|
|
|
CO2Me |
207 |
|
||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
MeO |
|
205 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
H |
|
CN |
|
|
|
|
|
|
|
CO2Me |
|
|
|
|
|
MeO |
206 |
|
|
|
|
|
|
Scheme 6.82 |
|
|
|
|
|
|
|
CN |
|
|
CN |
|
CN |
NC |
|
|
|
||
|
hν |
∆ |
|
|
|||
|
|
|
|
||||
|
|
+ |
|
|
|
|
|
N OEt |
|
N OEt CN |
N |
CN |
|||
208 |
|
|
209 |
210 |
|
|
OEt |
|
|
|
211 |
||||
|
|
|
|
|
|
|
hν |
|
|
|
|
|
|
|
CN |
|
|
|
|
|
|
|
CN |
|
|
|
|
|
|
N |
OEt |
|
|
|
|
|
|
212 |
|
Scheme 6.83
Special Topic 6.9: Photochemistry of fullerenes
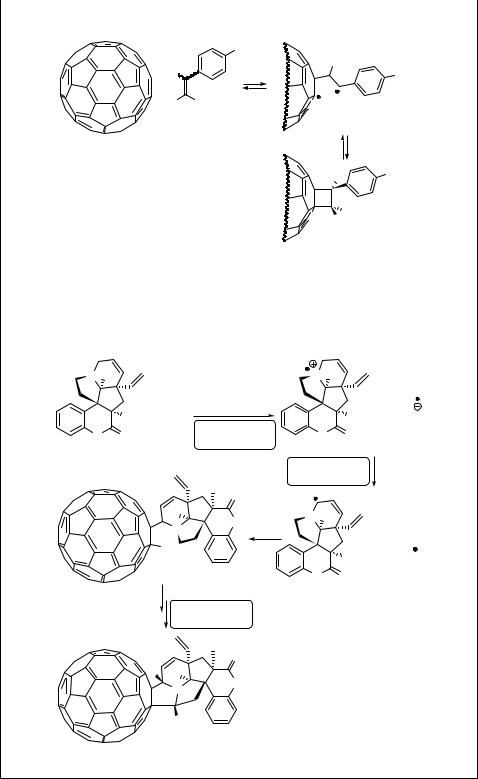
The (photo)chemical and physical properties of fullerenes, spherical carbon molecules, are an important topic in current research, especially in nanotechnology.823,824 The
molecule with 60 carbons, C60, having properties of an electron-deficient alkene, undergoes for example [2 þ 2] photocycloaddition similar to aromatic moieties. The absorption spectrum of C60 exhibits three principal maxima (lmax ¼ 211, 256, 328 nm) and a weak band at l ¼ 540 nm.813 Very weak fluorescence from the lowest excited singlet state (S1; ES ¼ 193 kJ mol 1) competes with fast and efficient (close to 100%) intersystem crossing to the excited triplet state (ET ¼ 157 kJ mol 1). For example, the cycloaddition of both (Z)- and (E)-1-(4-methoxyphenyl)prop-1-ene (213) to triplet excited C60 (214) leads to an adduct 215 through a biradical intermediate 216


 cycloaddition
cycloaddition 
 +
+ 
 O
O