
Photochemistry_of_Organic
.pdf
316 |
Chemistry of Excited Molecules |
carboxylic acids and optically pure amines crystallize in homochiral space groups. The ammonium ions thus act as chiral auxiliaries and the diastereomeric transition states
are differentiated by a chiral reaction medium, thereby leading to asymmetric induction.590,958 For the system shown in Scheme 6.120, irradiation of solid 279a,
employing (R)-( þ )-1-phenylethylamine as a chiral auxiliary, afforded an alkene 280 in 32% enantiomeric excess (ee), whereas irradiation of 279b [employing (S)-( )-1- phenylethylamine] produced the opposite enantiomer in 31% ee at >99% conversion. Photolysis of both 279a and 279b in methanol solutions led, of course, to racemic photoproducts only.
Experimental details: liquid-state photolysis.957 Acetonitrile solutions of 279c
(0.07 M) in Pyrex tubes were purged with nitrogen for 15 min and then irradiated with a medium-pressure mercury lamp (450 W) in a water-cooled Pyrex (lirr > 280 nm) immersion well (Figure 3.9) at 20 C for several hours. The chemical yields of 280 and 281 were 53% and 44% (GC), respectively.
Experimental details: solid-state photolysis.957 A crushed crystalline ketone (279a or 279b) ( 5 mg), suspended in hexane (3 ml), was placed between Pyrex microscope slides, sealed in a polyethylene bag under nitrogen and irradiated with a mediumpressure mercury lamp (450 W) at a distance of 10 cm from a water-cooled Pyrex immersion well (Figure 3.9) at either 20 or 20 C (cryostat ethanol bath). The product, a chiral organic salt, was derivatized to the corresponding methyl ester by treatment with excess diazomethane and purified by column chromatography.
6.3.5Carbonyl Compounds: Photocyclization Following n,1-Hydrogen Abstraction
O |
H |
OH |
R |
(CH2)x |
R (CH2)x |
Recommended review articles.751,863,933–935,959–961
Selected theoretical and computational photochemistry references.942,945
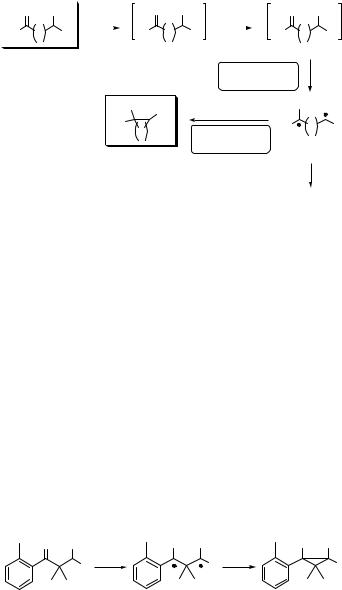
Excited carbonyl compounds containing g-C H bonds undergo characteristic intramolecular hydrogen abstraction reactions to yield both cleavage (Norrish type II
reaction;917Section 6.3.4) and cyclization (Yang photocyclization947) products via 1,4- biradical intermediates (Scheme 6.121).863,959-961 In the absence of reactive g-hydrogens,
intramolecular hydrogen abstraction from a non-g-position and subsequent cyclization reaction may also occur.
The initial step in a photocyclization reaction is intramolecular hydrogen abstraction by an excited carbonyl, basic details of which we described in Section 6.3.4. The g-hydrogen
abstraction, proceeding via a six-membered ring transition state, is energetically favoured.933,938,960,961 In contrast, the b-hydrogen transfer is disfavoured for enthalpic
reasons because of the strain that develops in the corresponding five-membered transition state permitting the abstraction, whereas a more negative entropy associated with the larger cyclic transition states (d- or more distant hydrogen abstraction), reflects the relative

320 |
Chemistry of Excited Molecules |
Long-distance Hydrogen Transfer: Formation of Sixand Larger Membered Rings
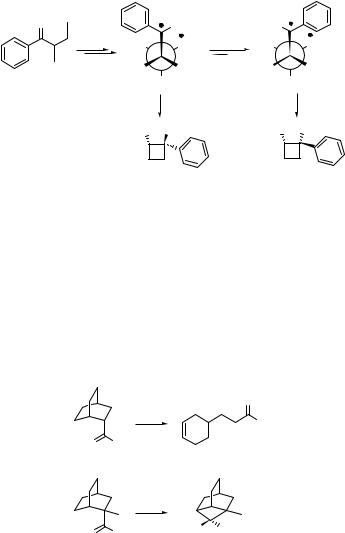
There are few experimental examples of longer distance intramolecular hydrogen abstraction. e-Hydrogen transfer forming 1,6-biradicals generally requires that g- and d-hydrogen atoms be unavailable or unreactive [as, for example, in the b-(o-tolyl) propiophenone 291 (Scheme 6.127)].968
HO
R |
hν |
R |
O
291
Scheme 6.127
The photochemically induced reaction of 292 is an example of seven-membered ring formation, which gives the product 293 in 25% chemical yield (Scheme 6.128).969 The mechanism involves a spin centre shift960 approach, which is based on the formation of acommon biradical and a subsequent efficient rearrangement bypassing the otherwise favourable cyclization. This reaction, like cyclopropane ring opening (see also Special Topic 6.10), enables one of the radical centres to shift, hence creating a new, more remote biradical that eventually cyclizes.
|
O |
|
O |
|
Ph |
|
Ph |
O |
hν |
|
O |
Ph |
O |
Ph |
OH |
292
spin centre shift
|
O |
|
O |
Ph |
|
O |
O |
|
OH |
OH |
|
Ph |
||
|
||
Ph |
|
|
293 |
Ph |
|
|
Scheme 6.128
Special Topic 6.14: Photochemical synthesis of large rings
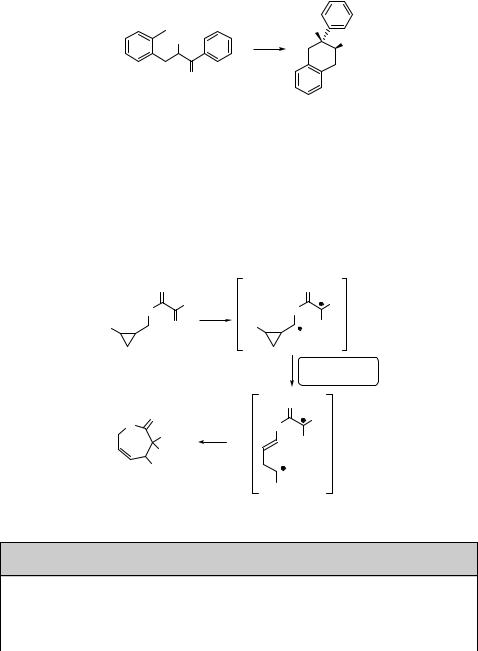
Photocyclization following 1,n-hydrogen abstraction seems to be an excellent tool for the synthesis of large macrocycles – one of the great challenges in organic synthesis. Irradiation of benzyloxypentyl phenylglyoxylate (294), for example, gives 3-hydroxy- 3,4-diphenyl-1,5-dioxacyclodecan-2-one (295) in 20% chemical yield (Scheme 6.129),

Oxygen Compounds |
323 |
derivative 301, followed by reduction of the oxo intermediate 302 (Scheme 6.132).973 The cyclization step obviously proceeds via the ketyl radical anion formed by electron transfer from triethylamine to the excited ketone.876
O |
|
HO |
HO |
hν, Et3N |
|
|
|
|
|
|
|
N |
CH3CN |
N |
N |
O |
|
H O |
H |
|
|
|
|
301 |
|
302 |
300 |
Scheme 6.132
Experimental details.973 An acetonitrile solution of 301 in a 10 mm quartz tube, purged with argon for 30 min, was irradiated in a Rayonet system (Figure 3.10) using 12 low-pressure mercury lamps emitting at lirr ¼ 254 nm. The product was obtained in 46% yield.
6.3.6Carbonyl Compounds: Photoenolization
Recommended review articles.939,959,974
Selected theoretical and computational photochemistry references.975–977
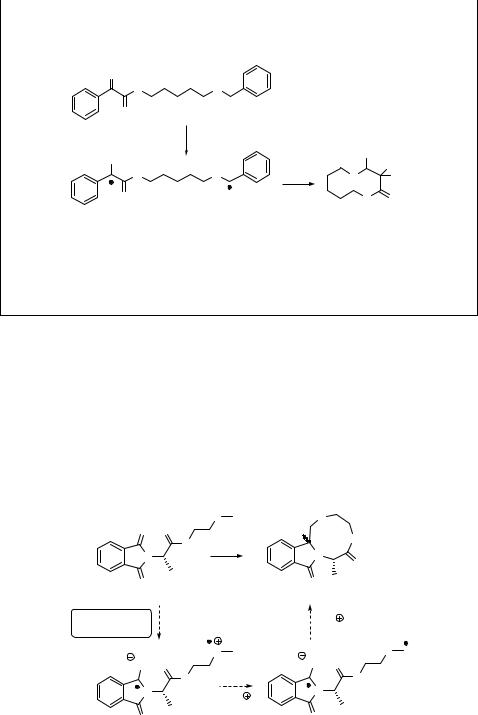
2-Alkylphenyl ketones are known to produce readily the corresponding enols (photoenols) upon photochemical excitation.974,978 For example, 2-methylacetophenone (303) undergoes intramolecular 1,5-hydrogen abstraction via the triplet state to form a triplet 1,4-biradical (triplet enol), yielding two isomeric photoenols, E- and Z-, whereas
fast direct enolization from the lowest excited singlet state produces the Z-isomer only (Scheme 6.133).979,980 The Z-isomer, having a lifetime similar to that of the biradical, is
converted efficiently back to the starting molecule, but the E-isomer may, in the absence of trapping agents such as dienophiles, persist for up to seconds because its reketonization requires proton transfer through the solvent. This reaction can be accompanied by other photochemical reactions typical for the excited ketones, such as hydrogen abstraction (Sections 6.3.1 and 6.3.4).
The cyclization reaction of a biradical intermediate to form cyclobutanol (304; see also
Yang cyclization; Section 6.3.5) and the Diels–Alder cycloaddition of the photoenols with dienophiles are common subsequent processes (Scheme 6.134).981,982 Both processes are
often stereospecific, typically involving a single, long-lived (E)-photoenol.
Oxygen Compounds |
325 |
photoisomerization (Section 6.1.1), also undergo intramolecular cyclization to yield the corresponding product 306 (Scheme 6.135). Irradiation of (E)-305 led to syn- and anti-306 in a concentration ratio of 25:1, whereas irradiation of (Z)-305 provided a reverse ratio (1:3). These results understandably depended on the photostationary state (Section 3.9.4) concentrations of the isomers of 305. It was concluded that the minimization of steric repulsion between the methyl and hydroxymethyl groups anti to one another in the transition state (represented by 307) must be responsible for the formation of a single racemic product.
|
O |
O |
O H |
OEt |
O H |
|
EtO |
|
|
hν |
|
OH |
hν |
OH |
(E)-305 |
|
(Z)-305 |
hν |
|
hν |
OH O |
|
OH O |
|
OEt |
OEt |
H |
|
H |
HO |
|
HO |
syn-306 |
|
anti-306 |
|
OH |
O |
|
|
OEt |
HO
307
Scheme 6.135
Experimental details.983 A benzene solution of 305 (0.08 M) in an argon-purged Pyrex vessel was irradiated using a medium-pressure mercury lamp (450 W) in an immersion photochemical reactor (Figure 3.9) for 20–40 min. The solvent was then evaporated and the reaction mixture was separated by column chromatography to give the product in 90% chemical yield.
When leaving groups are present in an appropriate position, the primary photoenols can undergo elimination reactions. For example, leaving groups such as chloride984,985
or carboxylate986–988 on the a-carbon of 2-methylphenacyl compounds are efficiently released to form the indanone derivatives 308 in non-nucleophilic solvents. Furthermore, the acetophenone derivatives substituted on the o-methyl group 309

 R
R