
Photochemistry_of_Organic
.pdf
406 |
Chemistry of Excited Molecules |
type I oxygenations.1365,1366 In such a case, a radical 498, typically formed via hydrogen atom transfer between an excited species (sensitizer) and an organic hydrogen donor, is trapped by oxygen to form a peroxy radical intermediate 499, which undergoes subsequent reactions (Scheme 6.245).1365 In contrast, photoinduced electron transfer from an electron donor, such as an radical anion 500, to ground-state oxygen can give a reactive superoxide anion (O2. ; 501), which readily reacts with the radical cation 502 formed in the first step (Scheme 6.246).1366
sensitizer* + |
substrate |
|
H |
|
|
sensitizer |
|
H |
|
+ substrate |
||||||
|
|
|
|
|
||||||||||||
|
|
|
||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
498 |
|
substrate + |
3O2 |
|
|
|
substrate |
|
O-O |
|
|
|
oxidation products |
|||||
|
|
|
|
|
|
|||||||||||
|
|
|
|
|
|
499 |
|
|
|
|
|
|
||||
Scheme 6.245
sensitizer* + substrate  sensitizer
sensitizer + substrate
+ substrate
|
|
|
500 |
502 |
sensitizer |
+ |
3O |
sensitizer |
+ O |
|
|
2 |
|
2 |
|
|
|
|
501 |
substrate |
+ |
O2 |
oxidation products |
|
Scheme 6.246
Special Topic 6.21: Atmospheric photochemistry
Photochemical reactions have played a decisive role in the evolution of the atmosphere and of life on Earth. Such processes generally involve simple species, many of which are otherwise considered to be stable and unreactive. Special Topic 6.17 has already discussed photochemistry in a prebiotic atmosphere. Since geochemical inorganic processes cannot be responsible for the current level of oxygen in the atmosphere (21%), it must be almost exclusively the product of biological activity (Special Topic 6.25).
There are two principle photoreactions connected to atmospheric oxygen.1367 When stratospheric (approximately 15–35 km above the Earth s surface) molecular oxygen is exposed to UVC (l < 240 nm), oxygen atoms are produced and react with
O2 to form ozone. This important compound strongly absorbs UVB (l < 300 nm) radiation (Figure 1.1),1368 so that only a small part of the life-threatening UV
radiation from the Sun reaches the Earth s surface:
O2 þ hn ! 2O
O þ O2 ! O3
O3 þ hn ! O2 þ O

Molecular Oxygen |
407 |
Ozone in the stratosphere is depleted by reactions with halogen atoms. Depletion of stratospheric ozone, commonly referred to as the ozone hole, usually occurs over the Earth s cold regions. The main source of chlorine atoms in the stratosphere is photodissociation of chlorofluorocarbon (CFC) compounds,1369 commonly called
Freons, e.g.:
CFCl3 þ hn ! .CFCl2 þ Cl.
O3 þ Cl. ! O2 þ ClO.
Nitrogen oxide, formed by photofragmentation of NO2 or chlorine nitrate (ClONO2), hydroxyl radicals and some other reactive species are also responsible for stratospheric ozone depletion. These compounds may have both natural and anthropogenic
(combustion, etc.) origin. Atmospheric chemistry also takes place in aerosol particles, cloud droplets1370 and ice crystals.1371,1372
Ozone depletion in the stratosphere (causing enhanced levels of the penetrating UV radiation) leads to increased tropospheric ozone levels. Ground-level ozone can pose a health risk, because it is a strong oxidant. Furthermore, other airborne tropospheric pollutants, such as SO2, NO2 and HNO2, are also photolabile and may be responsible for the formation of secondary pollutants.1367 Hundreds of individual volatile organic compounds (VOCs), such as alkanes, alkenes, aromatic hydrocarbons, oxygen and nitrogen-containing compounds, are important trace constituents of the atmosphere. Photochemical reactions also participate in their production.1373 For example, nitrous acid undergoes rapid photolysis after sunrise (in so-called photochemical smog1374),
which leads to early-morning production of hydroxyl radicals. They may abstract hydrogen from VOCs,1375 such as methane, to give a radical, which reacts with
oxygen and subsequently with NO to form formaldehyde:
HNO2 þ hn ! HO. þ NO.
CH4 þ HO. ! .CH3 þ H2O
.CH3 þ O2 ! H3COO.
H3COO. þ NO ! H3CO. þ NO2
2H3CO. þ 1=2 O2 ! 2H2CO þ H2O
Other harmful compounds, such as peroxyacyl nitrates (PANs), respiratory and eye irritants, are also produced in photochemical smog:
hydrocarbons þ O2 þ NO2 þ hn ! RCðOÞOONO2
Molecular Oxygen |
409 |
Special Topic 6.22: Phototoxicity and photoallergy
Photosensitivity reactions are adverse skin responses to the combined action of a new chemical (such as a drug) and light.1381,1382 The mechanisms are usually complex but
we can distinguish two types of reactions: phototoxic and photoallergic. More common phototoxic reactions result from direct tissue damage caused by in situ formation of singlet oxygen. In contrast, a photoallergy is an immunologically mediated hypersensitivity developed to a small amount of photochemically produced compound. The list of chemicals associated with photosensitivity includes several common antibiotics, such as sulfonamides and tetracyclines, diuretics, tranquillizers, cancer medicines and psoralen (Special Topic 6.8), fragrances, and so on. Most phototoxic agents absorb radiation in the UVA range.
For example, tetracycline (506), a broad-spectrum antibiotic, and its derivatives are known to induce phototoxic or photoallergic reactions that involve photosensitization of biomolecules by the drug or the formation of one or more photoproducts and their subsequent photoreactions.1383 Singlet oxygen is probably involved.
Cl OH |
N(CH3) |
H |
H |
|
OH |
NH2
OH O HOHO O O
506
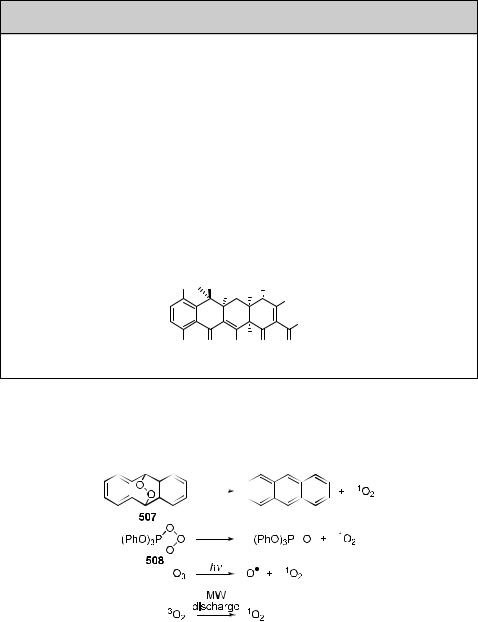
Singlet oxygen can also be obtained by thermal decomposition of unstable molecules, such as arene endoperoxides (e.g. 507) (Section 6.7.2) or ozonides (e.g. 508),135,1384,1385 by photolysis of ozone1386 or arene endoperoxides (such as 507),1384,1387 or by microwave
discharge in the presence of ground-state oxygen (Scheme 6.248).1388
∆ 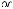
∆
Scheme 6.248
Singlet oxygen is involved in many important chemical processes and photochemical applications, including photodynamic therapy (Special Topic 6.23), photocarcinogeneity (Special Topic 6.7) and phototoxicity (Special Topic 6.22), chemiluminescence (Section 5.6), atmospheric photochemistry (Special Topic 6.21), polymer degradation (Special Topic 6.13), photosynthesis1389 (Special Topic 6.25) or industrial organic synthesis (Special Topic 6.20).


|
Molecular Oxygen |
411 |
H2N |
|
|
|
NH |
N |
O |
in vivo |
|
|
|
|
|
N |
HN |
COOH |
|
|
|
HOOC(H2C)2 |
(CH2)2COOH |
511 |
512 |
|
Scheme 6.249
Singlet oxygen, generated in an essentially heterogeneous biological cell, can be quenched with the biomolecules in the vicinity, resulting in cell killing through controlled cell death (apoptosis)1401 or necrosis, associated with loss of plasma membrane integrity.1400 There are many molecular targets of photodynamic effect in biological systems, including aromatic and sulfur-containing amino acids, unsaturated lipids, steroids and guanosine nucleotides, that undergo various oxidation reactions (type II photochemistry; see also the following sections).1391 The type I mechanism, according to which the excited triplet sensitizer participates in electron transfer reactions with molecules in the vicinity to produce free radical intermediates that react with ground-state oxygen to produce peroxy radicals and other reactive oxygen species (Scheme 6.246), is believed to be far less important.1400
The extent of the photodynamic effect is connected particularly to the lifetime of singlet oxygen and its concentration in a cell. Contrary to earlier reports, it has been found to diffuse over appreciable distances and across cell membranes into the extracellular environment.1378 Using a direct detection method called singlet oxygen microscopy, which has been developed to generate images of singlet oxygen (timeresolved) phosphorescence in a range of materials including single cells, the singlet
oxygen lifetime in a cell has been determined to be about 3 ms, allowing for its diffusion over approximately 130 nm.1402
Special Topic 6.24: Environmental aquatic and snow photochemistry
Sunshine can initiate photochemical reactions in atmospheric water droplets, in surface
and marine waters and even in snow. Solar short-wavelength light can reach ecologically significant depths in freshwater and marine ecosystems.1367,1403 In coastal
waters with high turbidity and larger concentrations of yellow matter , a naturally occurring complex mixture of coloured organic compounds collectively called humic acids, UVB (280–315 nm) penetrates only tens of centimetres, whereas penetration to depths of tens of metres can occur in clear oceanic waters.1404 Solar UV radiation at current or potentially enhanced levels due to stratospheric ozone depletion (Special Topic 6.21) has been found to affect, either positively or adversely, most forms of life found in shallow waters. A large proportion of dissolved organic material (DOM), including compounds of anthropogenic origin, absorb in the wavelength range

414 |
Chemistry of Excited Molecules |
Possible side-reactions, such as the formation of allyl hydroperoxides from compounds
having an allylic hydrogen (a competitive ene reaction; Section 6.7.3) or 1,4-endoperoxides from 1,3-dienes, may be suppressed by changing the experimental conditions.1359,1438 For
example, the extent of [2 þ 2] and [4 þ 2] photooxygenation in styrenes, such as the propenylanisole 522 (Scheme 6.253), is controlled by solvent polarity and pH, possibly due to protonation of a perepoxide/zwitterion intermediate.1421,1439 The [4 þ 2] product 523 is preferentially produced in non-polar benzene or chloroform, whereas the 1,2-dioxetane 524 is almost exclusively formed in methanol or acidified non-polar solvents.
R |
|
+ 1O2 |
|
|
|
|
|
522, R = OMe |
|
|
|
|
|
|
O |
R |
|
|
|
|
R |
O |
OO |
R |
|
||
|
H |
|
|
|
H O O |
|
|
|
|
|
523 |
||
|
|
|
|
|
||
|
OH |
R |
|
|
O |
|
R |
O |
OOH |
R |
|||
O |
||||||
|
|
|
|
- H |
||
|
|
|
|
524 |
||
|
|
|
|
|
Scheme 6.253
1,2-Dioxetanes such as tetramethyl-1,2-dioxetane (525) are known to undergo thermal
decomposition to form two carbonyl compounds via a concerted or stepwise (radical) mechanism, accompanied by chemiluminescence (Section 5.6) (Scheme 6.254).135,511,1440
The degradation of 525 results principally in acetone phosphorescence (lmax ¼ 430 nm) and the reaction is very sensitive to quenching by oxygen.
O O |
∆ |
O* |
O |
|
|
O |
525 |
|
|
+ |
|
2 |
+ hν |
|
|
|
||||
|
|
|
|
|
|
Scheme 6.254
Dioxetanes are useful precursors to various oxidation products, such as carbonyl and carboxyl compounds. Photooxidative double bond cleavage, an alternative to ozonolysis, can successfully be adopted for the preparation of large heterocyclic rings or for ringopening procedures. For example, photooxygenation of enamine 526 leads to the 10-membered product 527 in 84% chemical yield via a dioxetane intermediate 528 (Scheme 6.255).1441
In another example, the dioxetane 529, obtained from the oxathiin 530 by irradiation in the presence of oxygen and tetraphenylporphyrin (TPP), is converted to 531 in nearly quantitative chemical yield (Scheme 6.256).1442

