386 |
Chemistry of Excited Molecules |
6.5.2Sulfones, Sulfonates and Sulfoxides: Photofragmentation
R R' |
hν |
R |
R' |
R |
R' hν |
R |
R' |
S |
|
S |
S |
|
S |
O O |
|
O O |
|
O |
|
O |
|
Recommended review articles.1277–1279
Selected theoretical and computational photochemistry references.1280,1281
The photochemistry of sulfones (e.g., 4511282) and sulfonates (e.g. 4521283) is dominated by homolytic fissions of the S–C or S–O bonds (Scheme 6.214).1277,1278 Such
bonds are relatively weak; for example, the dissociation energy (DS–C) in CH3SO2–CH3 is 280 kJ mol 1 and DS–O in HO–SO2CH3 is 306 kJ mol 1.
|
|
|
CH3 |
hν |
|
H3C |
|
Ph |
Ph |
+ |
S O |
|
S |
|
|
|
O O |
S-C bond |
|
|
O |
|
|
451 |
fission |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ph |
|
|
|
|
|
|
S-O bond |
S O |
+ |
O CH3 |
|
Ph |
O |
hν |
O |
|
|
|
fission |
|
|
|
|
S |
CH3 |
|
|
|
|
|
O O |
|
|
|
HO |
|
|
|
452 |
|
|
|
|
|
|
|
S-C bond |
Ph + |
|
S O |
|
|
|
|
|
O |
|
|
|
|
|
fission |
|
|
|
Scheme 6.214
Aliphatic sulfones absorb only at l < 200 nm, whereas aromatic sulfones can be irradiated at 250–300 nm. Photolysis of sulfones is a convenient source of alkyl or aryl radicals, which may subsequently undergo various secondary reactions. The SO2 photoextrusion1284 can originate from both the singlet and triplet excited states depending on the sulfone substituents and the photoreaction can also be triplet sensitized. For example, photofragmentation of 453 to give the cyclophane 454 (54% chemical yield) proceeds primarily from the excited singlet state, whereas that of 455 gives a racemic mixture of the products [( )-456; 64% chemical yield] from the excited triplet state (Scheme 6.215).1285 This manner of producing cyclophanes is practical because the parent sulfones are easily prepared.
The photochemistry of sulfonates involves the excited singlet state and homolytic cleavage of either the S–C or the S–O bond (Scheme 6.214).1283 Alkylsulfonates absorb light only at shorter wavelengths (lmax < 200 nm), but quartz-filtered irradiation (lirr > 250 nm) is sufficient for excitation of arylsulfonates. Direct irradiation of
|
Sulfur Compounds |
387 |
O2S |
SO2 |
hν |
S * |
|
|
|
|
453 |
|
|
454 |
O2S |
SO |
hν |
T * |
|
ISC |
|
|
2 |
|
|
|
455 |
|
|
(±)-456 |
Scheme 6.215
p-toluenesulfonates, for example, leads to S–C bond homolytic cleavage in the first step, followed by SO2 extrusion (Scheme 6.216).1286 For example, the photoinduced removal of sulfonate moieties has been used in photodeprotection of the hydroxy groups in carbohydrates (see also Special Topic 6.18).1287
|
O |
|
|
|
|
|
|
O S |
|
|
|
|
|
|
O |
|
|
OH |
|
|
O |
O |
hν |
O |
O |
+ SO2 |
+ |
|
|
O |
|
|
O |
|
|
O |
O |
|
|
|
|
|
|
|
|
O |
|
|
O |
|
|
Scheme 6.216
Case Study 6.33: Photoremovable protecting groups – chemistry of carbohydrates
The p-tolylsulfonyl group can be utilized as a photoremovable group (Special Topic 6.18) for the protection of carbohydrates in the synthesis of saccharides in the presence of bases, such as the hydroxide anion or amines. No correlation has been observed between the nucleophilicity of the bases and the efficiency of the reaction;
however, a qualitative correlation was found with the electron-donating ability of amines.1288 It has therefore been suggested that the cleavage is induced by an
electron-transfer process via the excited singlet state of the sulfonate, the anion-
radical ArSO2OR. , and subsequent release of a leaving group (RO ) (Scheme 6.217).1288,1289

388 |
Chemistry of Excited Molecules |
hν
ArSO2OR  ArSO2OR
ArSO2OR  ArSO2 + OR Et3N
ArSO2 + OR Et3N
Scheme 6.217
For example, photolysis of methyl 3-O-benzyl-2,6-dideoxy-4-O-(p-tolylsulfonyl)- a-D-arabino-hexopyranoside (457) in the presence of a high concentration of triethylamine gives the glycoside 458 (Scheme 6.218).1289
O |
S O |
|
|
OH |
O |
|
|
BnO |
|
hν BnO |
|
|
O |
|
|
O |
|
Et3N |
|
|
|
OMe |
|
|
OMe |
457 |
|
|
458 |
Scheme 6.218
Experimental details.1289 A solution of 457 (0.74 mmol) and triethylamine (2 mmol) in methanol was purged with nitrogen for 1 h and irradiated in a Rayonet photochemical chamber equipped with 16 low-pressure mercury discharge lamps (254 nm) (Figure 3.10) for 12 h. After irradiation, methanol was distilled off under reduced pressure and the product was purified by column chromatography in 90% chemical yield.
Homolytic fission of the C S bond is a primary process following the excitation of sulfoxides. For example, the sulfoxide 459 produces a 1,5-biradical, which can either rearrange to the sulfenate ester 460 or revert to the starting material (Scheme 6.219).1290
|
|
hν |
|
|
|
|
S O |
|
S O |
hν |
S |
Ph |
Ph |
Ph O |
|
459 |
|
|
460 |
|
6.5.3Problems
1.Explain the following concepts and keywords: photofragmentation; photoextrusion.
2.Suggest the mechanisms for the following reactions:
(a)
[ref. 1291]
(b)
TMS |
|
SO2 |
H |
|
hν |
|
O |
O |
|
(TMS = trimethylsilyl) |
|
[ref. 1292]
3.Predict the major photoproduct(s):
(a)
S
hν
H
SH
[ref. 1293]
(b)
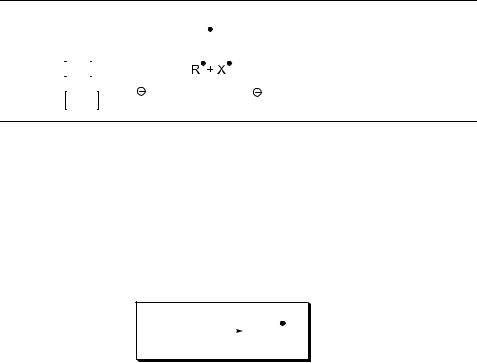
390 |
Chemistry of Excited Molecules |
6.6 Halogen Compounds
Halogen substitution has little effect on the absorption spectra of conjugated compounds. The heavier halogens bromine and iodine may, however, substantially increase the rate of ISC, especially when the parent compound has a long excited-state lifetime. The s,s absorption of haloalkanes reaches into the near-UV region, increasingly with the heavier halogens and geminal dior trisubstitution. Halogenated aliphatic solvents have relatively low cut-off wavelengths (see also Table 3.1), for example, 2,2,2-trifluorethanol (<190 nm),
dichloromethane ( 230 nm), chloroform ( 250 nm), carbon tetrachloride ( 265 nm) and bromoform ( 300 nm).158
In this section, we discuss separately the chemical reactions that follow photofragmentation of a halogen molecule and the reactions of excited halogen-containing organic molecules. In the first case, a light-absorbing halogen molecule, such as chlorine (Table 6.18, entry 1), is homolytically cleaved to give two halogen atoms, which take part in the subsequent radical chain reactions (halogenations) with, for example, saturated or unsaturated hydrocarbons. Since the dissociation energies of halogen molecules are relatively low, visible light is sufficient to promote the initial step.
Table 6.18 Photoprocesses involving halogen compounds
Entry |
Starting materiala |
Product(s) |
Mechanism |
Section |
|
|
|
|
|
|
|
|
1 |
X2* |
2X |
Photofragmentation |
6.6.1 |
2 |
|
R-X |
|
* |
|
Photofragmentation |
6.6.2 |
|
|
|
3 |
|
Ar-X * + Nu |
Ar-Nu + X |
Nucleophilic |
6.6.2 |
|
|
|
|
|
|
photosubstitution |
|
aX ¼ halogen atom; Nu ¼ nucleophile; Ar ¼ aryl.
Homolytic fission of the carbon–halogen bond is typical of excited organic alkyl and aryl halides (entry 2). Various subsequent reactions may then follow, including radical reactions such as hydrogen abstraction or rearrangements or electron transfer within the caged radical pair. The resulting ion pair intermediates can be attacked by nucleophiles, although direct nucleophilic attack on an excited aryl halide (see Scheme 6.93) is also possible (entry 3).
6.6.1Halogen Compounds: Photohalogenation
Recommended review articles.155,1295,1296
Selected theoretical and computational photochemistry references.1297–1301

Photohalogenation – a photoinitiated reaction between a halogen donor, such as a halogen molecule and a substrate – proceeds typically via a radical chain mechanism; therefore, the reaction quantum yield, closely related to the average chain length, is greater than unity.155
The absorption spectra of chlorine and bromine molecules have lmax at 330 nm (« ¼ 65 l mol 1 cm 1) and 420 nm (« ¼ 1651 l mol 1 cm 1), respectively.155 Since their
dissociation energies are 243 and 192 kJ mol 1, which correspond to photons of 492 and 623 nm wavelength, respectively, they readily undergo homolytic fission upon excitation, even when using visible (incandescent) low-power radiant sources.
In the photochlorination of alkanes, chlorine atom formation is followed by various propagation and termination radical reactions (Scheme 6.220). The hydrogen abstraction step is usually exothermic and irreversible. Hydrogen atoms are abstracted at rates that are in
the order primary < secondary < tertiary C H bonds; the activation energies for abstraction may vary from 4 kJ mol 1 in the first to 0.4 kJ mol 1 in the last case.155 Such small
differences indicate that selectivity disappears at higher temperatures. Hydrocarbon rearrangements are not common, but every possible monochlorinated product is usually formed.1295 The quantum yields may exceed 106 in the absence of radical trapping agents.
initiation
propagation
termination
Cl2 |
|
hν |
2 Cl |
|
|
|
|
|
|
Cl |
+ |
RH |
HCl |
+ |
R |
R |
+ Cl2 |
RCl |
+ |
Cl |
Cl |
+ |
Cl |
Cl2 |
|
|
Cl |
+ |
R |
RCl |
|
|
R |
+ |
R |
R2 |
|
|
R = alkyl
Scheme 6.220
Aromatic solvents and carbon disulfide often increase the chlorination selectivity due to the formation of p- or s-complexes between the solvent molecules and the chlorine atom. Such an interaction decreases the reactivity and hence increases the selectivity toward the hydrogen atoms. For example, photochlorination of adamantane (461) gives a higher production of 1-chloroadamantane in CS2 than in benzene (Scheme 6.221).1302
|
Cl |
|
|
hν |
|
|
Cl |
|
+ |
|
+ Cl2 |
|
|
461 |
68% |
: |
32% |
CS2 |
benzene |
54% |
: |
46% |
392 |
Chemistry of Excited Molecules |
|
|
hν |
Cl |
Cl |
Cl |
|
+ |
+ |
|
|
|
|
Cl2 Cl
462 463
Cl
Cl2  - Cl
- Cl
Cl
Scheme 6.222
Photochlorination of unsaturated hydrocarbons is initiated by exothermic addition of the chlorine atom to a multiple bond to form a chloroalkyl radical and is often accompanied by substitution and rearrangement reactions. This is demonstrated by chlorination of but- 2-ene (462), where the addition product, 2,3-dichlorobutane (463), is obtained as a major product (Scheme 6.222).1303
In contrast to photochlorination, the corresponding hydrogen abstraction or addition steps in photobromination are usually reversible, endothermic and more selective.155 The hydrogen abstraction rates in the allylic or benzylic positions can be relatively high, however. Table 6.19 shows the relative reactivities of the benzylic C H bonds in a series of alkylbenzenes in the photobromination reaction carried out in CCl4.1304 It is apparent that not only electronic but also steric effects control the reaction kinetics.
Table 6.19 Photobromination of alkylbenzenes
Alkylbenzene |
Relative reactivity |
|
|
|
[per hydrogen (H) atom]1304 |
|
H |
|
Ph |
|
|
1.0 |
|
|
|
|
|
|
H |
|
Ph |
|
CH3 |
17.2 |
|
|
|
|
H |
|
Ph |
|
Ph |
9.6 |
|
|
|
H |
|
Ph |
|
Ph |
|
Ph |
17.8 |
|
|
|
|
|
|
Case Study 6.34: Organic synthesis – photobromination of progesterone
The regioand stereoselective photobromination of progesterone (464), an a, b-unsaturated steroid ketone, gave the 6b-brominated product 465 (Scheme 6.223).1305 The reaction was carried out in the presence of cyclohexene oxide to trap HBr formed during the reaction.

|
Halogen Compounds |
393 |
|
O |
|
O |
H |
|
hν, Br2 |
H |
H |
H |
CCl4 |
H H |
O |
|
O |
|
|
|
|
Br |
464 |
|
|
465 |
Scheme 6.223
Experimental details.1305 A solution of progesterone 464 (6 mmol), bromine (6 mmol) and cyclohexane oxide (9 mmol) in CCl4 (120 ml) was exposed to light from a tungsten lamp (incandescent; visible light; 100 W) (Figure 3.9). The product precipitated after 15 h and was filtered off. Column chromatography of the remaining filtrate gave an additional amount of the starting material. The overall chemical yield was 87%.
N-Bromosuccinimide (NBS) (466) is a common bromination agent, used mainly for substitution reactions in the allylic or benzylic positions. The mechanism involves excitation of NBS generating either a bromine atom (Scheme 6.224a) or a succinimidyl
radical1306 (Scheme 6.224b) as one of the chain propagation steps, depending on the solvent and conditions used and, to some extent, on the reactivity of the substrate.1307
|
O |
|
|
|
O |
|
|
|
initiation |
N |
Br |
hν |
|
N |
+ |
Br |
|
|
|
|
|
O |
|
|
|
O |
|
|
|
|
466 |
|
|
|
|
|
|
|
Br |
+ |
RH |
O |
HBr |
+ |
R |
|
O |
|
|
|
|
|
|
|
propagation (a) HBr |
+ |
N Br |
|
Br2 |
+ |
N H |
|
|
|
O |
|
|
|
|
O |
R |
+ |
Br2 |
|
RBr |
+ |
Br |
|
|
|
O |
|
|
|
|
O |
|
|
|
N |
+ RH |
|
|
NH |
+ R |
propagation (b) |
O |
O |
|
|
|
O |
|
O |
R |
+ |
N |
Br |
|
RBr |
+ |
|
N |
|
|
O |
|
|
|
|
|
O |
394 |
Chemistry of Excited Molecules |
Special Topic 6.20: Organic photochemistry in industry
Although organic photochemistry has experienced remarkable growth, applications of photochemical synthetic methods in the chemical industry have been mostly limited to radical reactions, such as photohalogenation (this section), photopolymerization (Section 6.8.1) and to some extent photosulfochlorination, photooxidation
(Section 6.7) and photonitrosylation, although some other reactions are also being used.155
Photochlorination of aromatic compounds, for example, can lead efficiently to fully chlorinated products. Benzene is converted to 1,2,3,4,5,6-hexachlorocyclohexane with F ¼ 2500 (Scheme 6.225).1308 One of the stereoisomers, lindane, is a well-known insecticide (banned today), which was produced and used globally in agriculture in annual amounts of 106 tonnes.1309
Scheme 6.225
In another example of an industrial process, cyclohexane photonitrosylation, leads to a nitrosocyclohexane, which is converted to caprolactam, a precursor to nylon-6 polymerization (Scheme 6.226).155
hν
NOCl  NO + Cl
NO + Cl
NO
- HCl
Scheme 6.226
Cholesterol can be converted to vitamin D photochemically from 7-dehydrocholes- terol (provitamin D) (Special Topic 6.4) and this procedure is still used in industry.616 Vitamin D can also be made by irradiating yeasts rich in ergosterol. In addition, vitamin A (retinol) (see Special Topic 6.1) is synthesized by E–Z photoisomerization (Section 6.1.1), sensitized by chlorophyll or other chromophores (Section 6.8) of its 11-cis isomer, which is produced industrially by conventional synthetic steps.1310
Rose oxide (467), a valuable perfume additive, is manufactured by an ene reaction (Section 6.7.3) of citronellol (468) (derived from lemon grass) with oxygen by
irradiation in the presence of Methylene Blue as a triplet photosensitizer (Scheme 6.227).1311,1312
OH |
hν |
HO |
OH |
|
|
Methylene Blue, O2 |
O |
468 |
|
|
467 |
Scheme 6.227
Photopolymerization, that is, a polymerization process initiated by photochemical production of the reactive species – initiators (Section 6.8.1), is largely used in industry. Polymers can be synthesized by photoinitiation, but can also be modified by photochemical cross-linking (photochemical hardening or UV curing; photoimaging; photolithography; see Special Topic 6.27).
The most important and widespread applications of photochemistry are, of course, photoimaging techniques, such as photography and xerography (Special Topic 6.32).1313 Some other large-scale applications, such as environmental remediation of anthropogenic pollutants by photocatalytic processes (Special Topic 6.28) and potential applications such as photoelectrochemical cells (Special Topic 6.31) and artificial photosynthesis (Special Topic 6.26), are also discussed in the following text.
6.6.2 Organic Halogen Compounds: Photofragmentation, Photoreduction
and Nucleophilic Photosubstitution
R-X |
hν |
R + X |
|
R-X |
hν, [H] |
|
|
R-H |
|
|
|
|
|
|
|
|
|
hν, Nu
R-X  R-Nu
R-Nu
Recommended review articles.836,838–842, 849,852,1314–1321
Selected theoretical and computational photochemistry references.1322–1333








 - Cl
- Cl
 NO + Cl
NO + Cl

 ArSO
ArSO ArSO
ArSO R-Nu
R-Nu