
Photochemistry_of_Organic
.pdf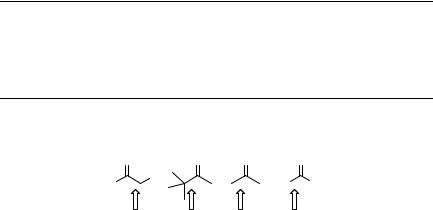
306 |
Chemistry of Excited Molecules |
Table 6.9 Quantum yields (FI) and rate constants of the Norrish type I cleavage (kI)a.
Ketone |
|
FI |
kI/108 s 1 |
PhCH2 COPh |
|
0.4 |
0.02 |
t-Bu COCH3 |
b |
0.3 |
>10 |
t-Bu CO(4-BP) |
|
<0.001 |
<0.001 |
Cyclobutanone |
|
0.3 |
>10 |
Cyclopentanone |
0.2 |
2 |
|
aAdapted from refs 905 and 918. b4-BP ¼ biphenyl-4-yl.
|
O |
O |
O |
O |
|
Ph |
Ph |
|
Ph |
Ph |
|
|
|
||||
D |
= 298 |
330 |
352 |
397 |
kJ mol-1 |
C-CO |
|
|
|
|
|
Figure 6.6 Bond dissociation energies in ketones
of magnitude higher than that of the p,p triplet of biphenylyl tert-butyl ketone [t-BuCO (4-BP)]919 (Table 6.9). Energy barriers for a-cleavage in aliphatic n,p ketones in solutions range from nearly 0 to 65 kJ mol 1, depending on the ketone structure and the spin multiplicity of the excited state.920 The cleavage rate constants of singlet n,p excited ketones are usually high (kI ¼ 108–109 s 1) and they compare with those of intersystem crossing. In the case of cyclic ketones, the release of the ring-strain energy during a-cleavage increases the exothermicity of the process.912 Cyclobutanone ring opening is, therefore, faster than that of cyclopentanone (Table 6.9).
Triplet n,p -state cleavage reactions (typically for aromatic ketones, FISC of which is usually 1.0; Section 2.1.6) are more efficient due to the longer triplet lifetimes and relatively large cleavage rate constants. Furthermore, recombination of the primary triplet radical pair formed is spin forbidden, which allows the radicals to escape the solvent cage. The photochemical racemization of the chiral phenylpropiophenone 264, for example, was found to depend on a partitioning between in-cage radical recombination and diffusion rate constants (Scheme 6.110).921
The decarbonylation rates of acyl radicals, the primary a-cleavage intermediates, are also related closely to the stability of the corresponding alkyl radicals formed, and hence to the magnitude of the bond dissociation energies (DC CO).903 Fast CO release is observed for the benzyl and tert-butyl radicals, whereas the formation of alkyl and particularly unstable aryl radicals is exceedingly slow (Table 6.10). An unsymmetrical ketone PhCOCH2Ph, for example, efficiently produces benzoyl and benzyl radicals upon photolysis, which undergo subsequent reactions. Decarbonylation to release carbon monoxide does not occur in this case because the Ph CO bond cleavage is energetically unfavourable.
The Norrish type I reaction of acyclic and cyclic ketones in solution typically results in recombination, decarbonylation and disproportionation (hydrogen abstraction) products.903 For example, irradiation of di-tert-butyl ketone (265) in hexane solution provides nearly exclusively decarbonylation products from both the singlet and triplet
states (>90% chemical yield), whereas the carbonyl group-containing products are produced only in traces (Scheme 6.111).922,923


Oxygen Compounds |
309 |
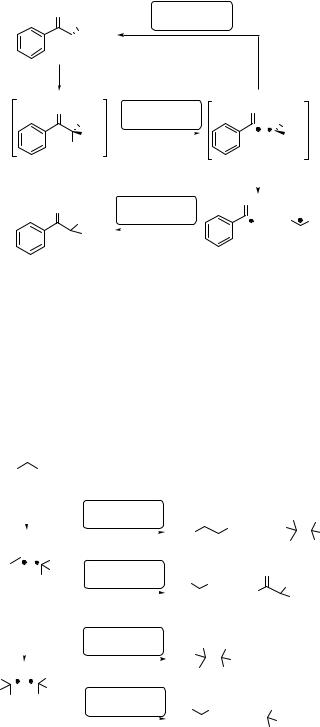
Case Study 6.19: Photochemistry in crystals – solid-to-solid photoreaction
Photochemical reactions in the solid state (see Special Topic 6.5) usually occur with high reaction selectivity and specificity due to conformational bias and the least-motion pathway principle.172,928 Photochemical decarbonylation of cis-2,6-dihydroxy-2,6- diphenylcyclohexanone (270) in homogeneous solution results in cis- and trans-271 cyclopentanediols in nearly equimolar ratio, whereas its photolysis in single crystals gives predominately the cis-isomer in a high chemical yield ( 83%) (Scheme 6.113).929 Free bond rotation in a biradical intermediate is largely restricted in the solid phase. An X-ray analysis revealed that the starting material and the cis-product have a high degree of structural similarity; therefore, the corresponding least-motion pathway reaction preserves shape and volume of the solid (crystalline) environment. By comparison, a small enhancement of cis-271 production was also observed in highly viscous sucrose glasses.
|
OH |
|
OH |
|
Ph |
HO |
hν |
HO |
|
HO |
|
|
Ph |
Ph |
free |
OH |
|
|
Ph O |
solution |
Ph |
Ph |
|
|
rotation |
||||
270 |
|
|
|
|
|
hν |
single |
|
|
|
|
crystal |
|
|
|
||
|
|
|
|
||
|
OH |
|
OH |
|
Ph |
|
HO |
|
HO |
|
HO |
|
Ph |
Ph |
|
OH |
|
|
Ph |
|
Ph |
|
Ph |
|
|
cis-271 |
|
|
trans-271 |
Scheme 6.113
Experimental details.929 Single crystals or fine powder of 270, placed between microscope slides, were irradiated with lirr ¼ 350 nm (Figure 3.9) at 20 C. Dissolution of the irradiated samples led to vigorous gas evolution, indicating that CO remained trapped in the crystal lattice. The ratio of photoproducts was determined by GC and NMR spectroscopy.
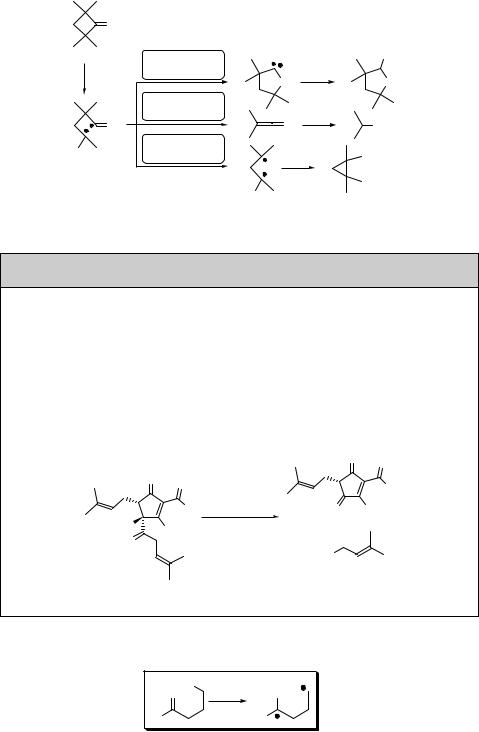
a-Cleavage in small-ring compounds releases the ring strain in the singlet state and can be sufficiently rapid to compete efficiently with intersystem crossing. Exhaustive photolysis of 2,2,4,4-tetramethylcyclobutanone (272) in methanol, for example, affords 2-methoxy-3,3,5,5-tetramethyltetrahydrofuran (273) as a major product, in addition to methyl isobutyrate and 1,1,2,2-tetramethylcyclopropane (Scheme 6.114).930 It has been suggested that the photoinduced ring expansion reaction involves an oxacarbene intermediate via the excited singlet state and a biradical intermediate.931
310 |
Chemistry of Excited Molecules |
|
O |
|
|
272 |
ring |
|
OMe |
hν |
expansion |
O |
MeOH |
|
O |
||
|
ketene |
|
273 |
|
|
|
|
|
formation |
O |
MeOH |
|
O |
COOMe |
|
|
decarbonylation |
|
|
Scheme 6.114
Special Topic 6.12: Photochemistry in beer
A skunky taste932 is known to occur upon exposure of beer to light. Photolysis of isohumulone (274) in methanol gives dehydrohumulinic acid (275) as a major reaction product (Scheme 6.115). Its formation apparently results from the a-cleavage of the 4-methylpent-3-enoyl group of 274 to yield 276, the compound which is believed to be largely responsible for the offensive beer flavour and odour. Because the iso-a-acids do not absorb in the visible region, the reaction is most likely due to sensitization by compounds, such as riboflavin (Section 6.8.1) and the presence of sulfur-containing amino acids (cysteine). Colourless and green bottles thus afford little protection from UV radiation; therefore, storage in brown glass bottles is advisable.
|
|
|
|
|
O |
O |
|
|
|
|
|
|
|
|
O |
O |
|
|
|
R |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
R |
hν (vis) |
O |
275 |
OH |
HO |
|
OH |
photosensitizer, |
|
|
|
|
|
+ |
|
|||
O |
|
a source of sulfur |
|
|
||
|
|
|
|
|||
|
|
|
|
|
||
|
|
|
|
HS |
|
|
|
274 |
|
|
|
276 |
|
Scheme 6.115
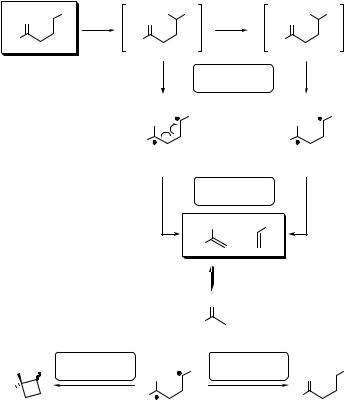
6.3.4Carbonyl Compounds: Norrish Type II Elimination
O |
H |
OH |
|
R |
R |
Oxygen Compounds |
311 |
Recommended review articles.751,863,933–939
Selected theoretical and computational photochemistry references.16,534,535,914,915,940–946
Many alkyl ketones with a g-hydrogen atom exhibit photoinduced intramolecular 1,5- hydrogen atom abstraction (Section 4.9) to form 1,4-biradicals (BR), which may undergo subsequent transformations, such as (1) reverse hydrogen abstraction to regenerate the starting material, (2) elimination to form an enol and alkene or (3) coupling of the
1,4-biradical triplet intermediate to produce cyclic alcohols (Yang photocyclization;947Section 6.3.5) (Scheme 6.116). 863,933–935,937 The elimination process is termed
the Norrish type II reaction.917 The a-cleavage to acyl and alkyl radicals, that is, the previously discussed Norrish type I reaction (Section 6.3.3), is usually suppressed.
|
2 |
1 |
2 |
* |
3 |
|
2 |
* |
O |
R |
|
H R |
|
|
H R |
||
hν |
|
O |
|
ISC |
|
O |
|
|
R1 |
|
|
R1 |
|
ΦISC |
R1 |
|
|
R1 = alkyl, aryl |
|
1,5-hydrogen |
|
|
|
|||
R2 = various groups |
|
|
|
transfer |
|
|
|
|
|
|
|
R2 |
|
|
|
R2 |
|
|
|
|
OH |
|
|
|
OH |
|
|
|
|
R1 |
|
|
|
R1 |
|
|
|
|
singlet BR |
|
|
|
triplet BR |
|
|
|
|
Norrish type II |
|
|
|||
|
|
|
1Φ II |
elimination |
|
3Φ II |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
OH |
R2 |
|
||
|
|
|
R1 |
|
+ |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
R1 |
|
|
|
|
|
2 |
Yang |
|
R2 |
|
reverse |
|
R2 |
|
HO R |
cyclization |
|
H-transfer |
O |
||||
|
|
|
OH |
|
|
|
|
|
R1 |
Φcycl |
|
1 |
|
Φ rev |
|
1 |
|
|
|
|
R |
|
|
|
R |
|
BR
Scheme 6.116
Both singlet and triplet excited states and two electronic configurations n,p and p,p have been shown to display the type II reactivity.863,933 Singlet state reactions are fast but
their low quantum yield indicates that hydrogen abstraction866 is accompanied by radiationless decay to regenerate the starting material. Triplet state reactions are usually more efficient. Ketones with the lowest n,p state are more reactive than those with the lowest p,p state. The n,p state, with a localized unpaired electron in a nonbonding orbital
312 |
Chemistry of Excited Molecules |
of the more electronegative oxygen atom, is responsible for a radical-like reactivity that parallels that of alkoxy radicals.
Aliphatic ketones have n,p lowest singlets that intersystem cross to their n,p lowest
triplets at rates below 108 s 1, which is slow enough to allow singlet reactions to occur.933,948 Structural changes in an alkyl chain can affect the photokinetic behaviour; the
faster the hydrogen abstraction, the less efficient intersystem crossing (Table 6.11).
Table 6.11 Norrish type II elimination of various alkyl ketones (CH3COCH2CH2R1)a
Ketone |
1FII |
1kH /108 s 1 |
FISC |
3FII |
3kH/108 s 1 |
|
1 |
¼ CH3 |
0.025 |
1.0 |
0.81 |
>0.36 |
0.13 |
R1 |
||||||
R |
¼ CH2(CH3)2 |
0.07 |
20 |
0.18 |
0.17 |
3.8 |
aIn benzene.933,948 1FII and 3FII are the quantum yields of the type II elimination from the singlet and triplet state, respectively (Scheme 6.116); FISC is the intersystem crossing quantum yield; 1kH and 3kH are the rate constants of 1,5-hydrogen abstraction.
Intersystem crossing from the singlet to triplet state in alkyl aryl ketones is usually rapid (<1011 s 1) and FISC is generally close to unity.863,933 Electron-donating substituents on an
aromatic ring of triplet aryl ketones are known to decrease the hydrogen abstraction reactivity by inverting their of triplet n,p lowest energy levels into those of p,p . Table 6.12 demonstrates the percentage of n,p triplets in equilibrium with p,p triplets and their effect on the observed type II rate constants. In systems where the p,p state is only several kJ mol 1 below that of n,p , most of the measured reactivity still arises from low populations of the n,p triplet. The p,p triplets with a low spin density on oxygen are known to undergo the hydrogen abstraction, but 2–3 orders of the magnitude slower than that observed for n,p triplets.
Table 6.12 Norrish type II elimination of various aryl ketones (R1COCH2CH2R2)a
Ketone |
FISC |
3FII |
kobs/108 s 1 |
n,p (%) |
|
1 |
¼ Ph |
|
|
|
|
R2 |
1 |
0.35 |
0.07 |
99 |
|
R |
¼ CH3 |
|
|
|
|
1 |
¼ Ph |
|
|
|
|
R2 |
1 |
0.33 |
1.4 |
99 |
|
R |
¼ CH2CH3 |
|
|
|
|
1 |
¼ p-alkylphenyl |
|
|
|
|
R2 |
1 |
0.39 |
0.18 |
18 |
|
R |
¼ CH2CH3 |
|
|
|
|
1 |
¼ p-methoxyphenyl |
|
|
|
|
R2 |
1 |
0.14 |
0.006 |
1 |
|
R |
¼ CH2CH3 |
|
|
|
|
aIn benzene. Symbols as those in Table 6.11 (see also Scheme 6.116); kobs is the observed rate constant of the type II elimination.933,949
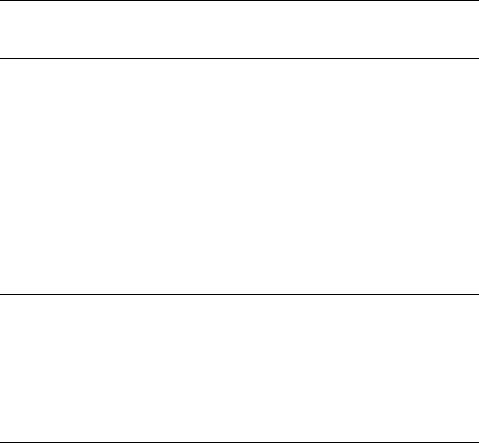
Several factors affect the partitioning among elimination, cyclization and reversion reactions of the biradical intermediates.933 Elimination (Grob fragmentation758) can occur from either gauche or anti conformations and this appears to be governed by the stereoelectronic requirement for overlap of the breaking bond with both half-occupied p-orbitals in the 1,4-biradical. Cyclization (and reversion) can take place from the gauche conformation only (Scheme 6.117). Singlet biradicals are generally too short-lived to allow for conformational changes; therefore, reversion and elimination are almost exclusively reactions of the singlet excited ketones in solutions. The longer lifetimes of

314 |
|
Chemistry of Excited Molecules |
|
|
Table 6.14 Temperature and environment dependence of the type II |
||
|
reaction in valerophenone |
|
|
|
|
|
|
|
Medium |
Temperature/ C |
E/Ca |
|
t-BuOH EtOH (9:1) |
20 |
7.9 |
|
t-BuOH EtOH (9:1) |
30 |
5.9 |
|
Silica gel surface |
125 |
Only C |
|
Zeolite ZSM-5 |
20 |
Only E |
aElimination/cyclization ratio.937
The type II reaction of n,p excited ketones is largely influenced by the reaction environment and experimental conditions.933,936,937 The initial step requires close
proximity of a g-hydrogen to the oxygen atom, i.e, overlap of the hydrogen s-orbital with the oxygen n-orbital. Flexible ketone chains achieve conformational equilibrium before g-hydrogen atom is transfered. Conformational (environmental) restrictions can suppress the subsequent elimination reaction and may tolerate other competing reactions, including the Yang photocyclization (Section 6.3.5). Specific inclusion of alkyl aryl ketones in zeolites or cyclodextrins, on the other hand, may prevent the cyclization reaction (Table 6.14).
Special Topic 6.13: Polymer photodegradation
Photodegradation of polymers (photoageing), involving chain scission and/or crosslinking, occurs by exposure to solar or artificial radiation and causes structural modifications, usually accompanied by a dramatic deterioration of the physical and mechanical properties of the polymer.954 Typically, radical intermediates are formed upon excitation, which initiate subsequent (dark) degradation. The presence of other species, such as oxygen, water, organic solvents or additives, and also mechanical stress and heat, may enhance the efficiency of these processes.
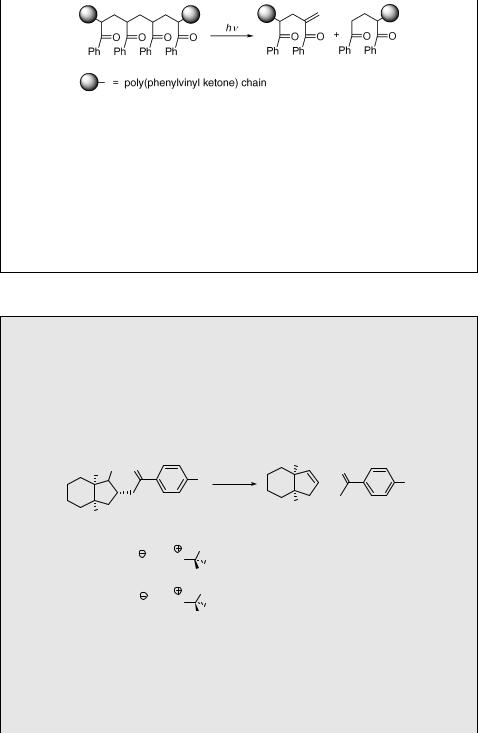
Many polymers, such as polyethylene and polystyrene, do not absorb above 300 nm. Their eventual photosensitivity is then attributed to unwanted incorporation of lightabsorbing and photoreactive species during manufacture and processing. When the chromophore is part of the polymer chain, as in poly(phenyl vinyl ketone), then direct photocleavage, in this case the type II process (Scheme 6.119), dramatically affects the polymer properties. The temperature-dependent stiffness of such a chain must then play the major role in obtaining the favourable chromophore geometry required for efficient g-hydrogen abstraction and cleavage.955 This compound is an example of a photodegradable polymer which is intentionally designed to become weak and brittle when exposed to solar radiation for prolonged periods. Photodegradable polymers degrade in a two-stage process; the photochemical reactions break specific bonds (some dark propagation steps may follow) to subsequently make the polymer brittle enough to degrade from physical stress. There are several potential applications, such as lowering the chemical persistence of polymers in the environment and photoimaging technology (see also Special Topic 6.27).

 CH
CH