326 |
Chemistry of Excited Molecules |
can be produced in the presence of a nucleophile (such as methanol) via (E)-photoenol (Scheme 6.136).
|
|
|
O |
|
|
|
308 |
|
non-nucleophilic |
- HX |
|
solvents |
|
|
O |
OH |
|
X |
|
|
X |
|
X |
OH |
hν |
|
+ |
|
|
|
X = -OCOR |
(Z)-photoenol |
(E)-photoenol |
|
|
MeOH - HX |
MeO
O
309
Scheme 6.136
Case Study 6.24: Photoremovable protecting groups – 2,5-dimethyl- phenacyl chromophore
Photoremovable protecting groups (PPG) (see also Special Topic 6.18) have found numerous applications in many fields of chemistry and biochemistry. The 2,5- dimethylphenacyl (DMP) chromophore cannot serve as a PPG for alcohols (which are relatively poor leaving groups) because their release is obviously too slow to compete efficiently with other processes, such as reketonization (Scheme 6.133). To overcome this problem, the alcohols can be attached via a carbonate link that possesses similar leaving group ability to that of a carboxylate. For example, the galactopyranosyl carbonate 310 was found to release the corresponding hydroxy-containing molecules in high chemical yields (<70%) and moderate quantum yields (0.1–0.5) (Scheme 6.137).989 The primary photoinitiated transformation liberates the carbonic acid 311 from a predominant (E)-photoenol, which subsequently slowly decarboxylates in the dark to release the corresponding alcohol 312.
Experimental details.989 A cyclohexane solution of 310 (0.005 M) in a Pyrex vessel, purged with argon, was irradiated using a medium-pressure mercury lamp (125 W) in an immersion photochemical reactor (Figure 3.9). The irradiation was stopped when the conversion reached at least 95%. The solvent was evaporated and the crude alcohol was purified by column chromatography.
|
|
Oxygen Compounds |
|
|
327 |
|
O |
|
|
|
O |
O R |
|
|
|
|
O O |
|
|
O |
O |
hν |
|
|
|
|
|
R |
|
H |
|
|
O |
|
cyclohexane |
|
|
|
|
|
|
|
|
310 |
|
|
(E)-photoenol |
R = |
O |
|
O |
|
HO |
O |
|
|
|
|
O |
|
|
|
O |
+ |
R |
|
O |
|
|
|
|
|
O |
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
311 |
|
|
|
|
|
|
|
|
|
|
|
CO2 |
+ HOR |
|
|
|
|
|
|
312 |
Scheme 6.137
6.3.7Quinones: Addition and Hydrogen/Electron Transfer Reaction
O |
hν, [H] |
OH |
|
|
O |
O |
|
O |
hν, [e ] |
O |
O |
O |
|
Recommended review articles990–995
Selected theoretical and computational photochemistry references.254,996–998
Both 1,2- and 1,4-benzoquinones and naphthoquinones are coloured compounds; 1,4-benzoquinone, for instance, displays two significant absorption band maxima above 250 nm (lmax ¼ 290 and 362 nm) and a weak absorption tail in the visible region. Intersystem crossing in quinones is generally fast and highly efficient and their photochemistry arises from the lowest excited triplet state in most cases.990,993,999 There is a significant difference in the reactivity of the 3n,p and 3p,p states. Hydrogen atom abstraction or cycloaddition of an alkene to the carbonyl group (oxetane formation; Section 6.3.2) occurs in quinones with low-lying 3n,p triplet states (Scheme 6.138). In contrast, cycloaddition reactions (Section 6.1.5) to the C¼C bond are observed for 3p,p excited compounds. The reactions can proceed by either direct irradiation or triplet sensitization.
328 |
|
|
|
|
|
|
|
Chemistry of Excited Molecules |
|
|
|
|
|
|
|
3n,π* |
|
|
|
|
|
O |
|
* |
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
oxetane |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
formation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
O |
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
[H] |
|
OH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1. hν |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
hydrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
abstraction |
|
|
|
|
2. isc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
π,π* |
|
|
|
|
|
O |
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
R |
|
|
|
|
cyclobutane |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
formation |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
O |
|
|
|
|
|
|
|
|
|
O |
Scheme 6.138
For example,3n,p excited 1,4-benzoquinone affords spiro-oxetanes in the presence of alkenes exclusively, whereas 1,4-naphthoquinone, in which the energies of the 3n,p and 3p,p states are close, produces both spiro-oxetanes and cyclobutanes.990 Introduction of electron-donor substituents on quinones further destabilizes the 3n,p state.
[2 þ 2] Photocycloaddition Reactions
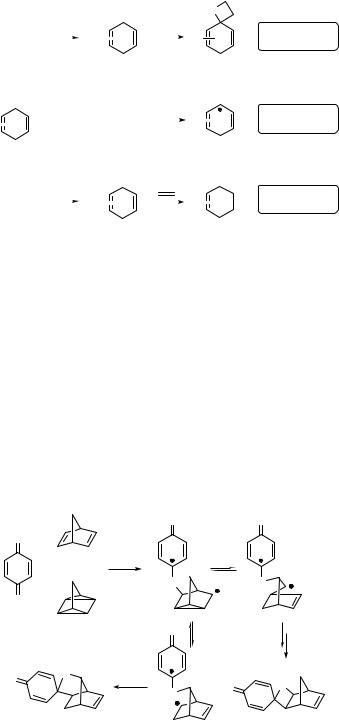
Cycloaddition reactions of triplet excited 1,4-quinones to ground-state alkenes occur either via a triplet exciplex intermediate, which collapses to a triplet biradical,1000 or via separated radical ion intermediacy.990 The existence of biradical intermediates has been proven by measurements of chemically induced dynamic nuclear polarization (CIDNP) (Special Topic 5.3), for example in the reaction of 1,4-benzoquinone (313) with norbornadiene (314) yielding two products, the spiro-oxetane 315 and the spiro-oxolane 316 (Scheme 6.139).1001 Interestingly, quadricyclane (317) provides the same reaction as norbornadiene.
|
|
O |
|
O |
O |
|
|
|
|
|
314 |
hν |
|
|
|
+ or |
|
|
|
|
|
|
|
|
O |
|
O |
O |
|
|
|
|
313 |
317 |
|
|
|
|
O |
|
|
|
|
|
|
O |
O |
|
|
O |
|
|
O |
|
|
O |
|
|
|
|
|
316 |
|
|
315 |
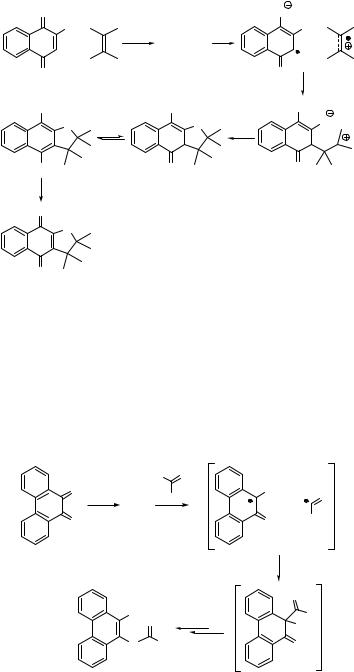
The hydroxy group at position 2 in 1,4-naphthoquinone (318) is involved in [3 þ 2] addition, producing the 2,3-dihydronaphtho[2,3-b]furan-4,9-dione 319 in 50% chemical yield (Scheme 6.140).1002 The reaction proceeds by a two-step process from a triplet excited quinone via exciplex (Section 2.2.3), intramolecular electron transfer (Section 5.2) and possibly ionic intermediate 320 formation.
|
O |
|
|
O |
|
OH |
hν |
|
OH |
|
+ |
exciplex |
+ |
|
|
|
O |
|
|
O |
|
318 |
|
|
|
|
OH |
|
OH |
OH |
|
O |
|
O |
O |
|
|
|
|
OH |
|
O |
O |
|
O2 (air) |
|
|
320 |
|
|
|
|
|
O |
|
|
|
|
O |
|
|
|
O
319
Scheme 6.140
Hydrogen Abstraction
Hydrogen abstraction is a typical reaction of triplet excited quinones.994 The first step involves hydrogen atom transfer to form a pair of radicals, similar to that occurring in simple triplet ketones (Section 6.3.1). Alternatively, hydrogen can also be abstracted in a two-step process, which is initiated by electron transfer from the hydrogen atom source to a quinone to form a radical ion pair, followed by proton transfer. The radical mechanism of this reaction is demonstrated in the following example: 9,10-phenanthrenequinone (321) in the triplet state abstracts the hydrogen atom from an aldehyde to form a triplet radical pair that recombines to give an acylated product 322 (Scheme 6.141).1003
|
|
|
H O |
3 |
|
O |
1. hν |
3321* |
R |
OH |
O |
|
|
|
|
+ |
O |
2. ISC |
|
O |
R |
321 |
|
|
|
|
|
|
|
|
|
|
ISC |
|
|
|
|
|
O |
|
|
OH |
|
|
R |
|
|
O |
|
|
OH |
|
|
|
|
|
|
|
O |
R |
|
O |
|
|
322 |
|
|
|
330 |
Chemistry of Excited Molecules |
Case Study 6.25: Green photochemistry – photochemical Friedel–Crafts acylation
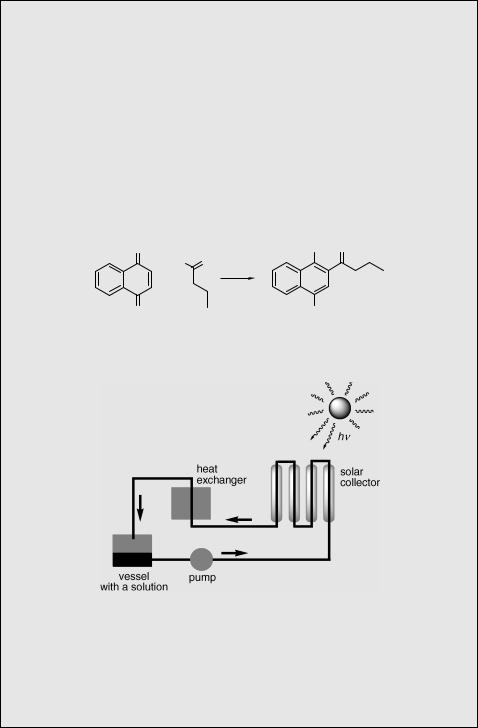
The synthetic significance of quinone photochemistry is demonstrated with the
photochemical Friedel–Crafts acylation 992,1004,1005 of 1,4-naphthoquinone (323) by butyraldehyde, which gives the 1,4-dihydroxynaphthalene derivative 324 via a hydrogen abstraction step and radical recombination (Scheme 6.142).1006 This reaction was carried out in a solar reactor, which consists of a reaction solution that circulates through a system of parabolic mirrors which collects light (lirr > 350 nm) and a heat exchanger to adjust the solution temperature (Figure 6.7). This photochemical reactor
(also called a solar plant) has been developed in the context of green photochemistry,1007,1008 which utilizes solar light as a renewable and cost-free source of
energy.1009,1010
O |
OH O |
H |
O |
+ |
hν |
|
O |
OH |
323 |
324 |
Scheme 6.142
Figure 6.7 Solar plant
Experimental details.1006 A solution of 323 (500 g) and an excess of butyraldehyde in a tert-butanol–acetone mixture, circulating in a solar reactor (Figure 6.7), was exposed to sunlight for 3 days to give 324 in 90% chemical yield and high purity (GC). The experiment took place in Spain during August and the total illumination time was 24 h.
6.3.8Carboxylic Acids and Their Esters: Photofragmentation and Rearrangement
hν
R-COOH  R + COOH
R + COOH
Recommended review articles.322,323,1011–1018
Selected theoretical and computational photochemistry references.1019-1024
Carboxylic Acids
The photochemistry of the carboxyl group has attracted only limited attention because it absorbs only at wavelengths well below 250 nm. The principle primary photoprocess is known to be either homolytic cleavage in the case of undissociated acids [for example, the dissociation energy (DC C) in CH3 COOH is 385 kJ mol 1] or heterolytic cleavage
in the case of carboxylate anions, followed by a photodecarboxylation step (Scheme 6.143).322,1025–1027 The latter process may involve the formation of a radical
cation precursor. The photoreactions of aliphatic derivatives usually proceed via an excited singlet state (the intersystem crossing efficiency in substituted acetic acids is low) and they are inefficient (F < 0.05).
|
hν |
|
R |
+ |
COOH |
|
|
|
|
|
|
|
O |
homolytic |
|
|
|
|
|
process |
|
|
|
|
|
|
R |
|
|
|
|
|
|
|
|
|
|
|
|
|
OH |
- H |
|
O |
|
hν |
|
|
|
|
|
R |
+ CO2 |
|
|
|
|
|
R |
|
|
heterolytic |
|
|
|
O |
|
|
|
|
|
|
|
process |
|
|
|
- e |
|
hν |
|
|
|
+ e |
|
|
|
|
|
|
|
|
R |
+ |
CO2 |
|
|
|
|
Scheme 6.143
For example, the photochemistry of non-steroidal anti-inflammatory drugs (NSAIDs) derived from 2-arylpropionic acid, has been studied because these compounds are known
to exhibit phototoxicity (see Special Topic 6.22) and skin photosensitivity in some patients.322,1011,1025 The photodecarboxylation of ketoprofen (325), a benzophenone
derivative, in neutral aqueous solution proceeds predominately via the excited triplet state (the aromatic ketone is the chromophore in this case) and a carbanion intermediate, formed
by intramolecular electron transfer (Scheme 6.144). The subsequent protonation yields the major product 326.1028,1029 The degradation quantum yields are higher from the
carboxylate form than from the non-dissociated acid. In addition, photoinduced hydrogen abstraction (Section 6.3.1) by the carbonyl group is also observed.
332 |
Chemistry of Excited Molecules |
|
O |
O |
|
COO 1. hν |
COO |
|
2. ISC |
[T1] |
|
|
|
325 |
- CO2 |
|
|
|
O |
O |
H2O
O
326
Scheme 6.144
Esters of Carboxylic Acids and Lactones
Aliphatic esters of carboxylic acids absorb below 200 nm;1013,1017 therefore, their photochemistry is usually associated with other chromophores. Photolysis of arylmethyl esters affords products derived from either homolytic or heterolytic cleavage via the excited singlet state (Scheme 6.145). The resulting radical or ionic intermediates readily undergo subsequent radical or polar reactions, respectively. The bond dissociation energies of the acyl [O CC(O)] bond are relatively low (e.g. DO C ¼ 355 or 313 kJ mol 1 for ethyl acetate or phenyl benzoate, respectively874); therefore the energy of photons with wavelengths below 330 nm is sufficient for s-bond homolytic fission.
|
R |
+ CO2 |
+ H2C Ar |
|
|
|
|
decarboxylation |
|
|
O |
|
|
|
R |
O |
+ H2C Ar |
hν |
homolytic |
|
|
process |
|
|
electron |
|
|
|
|
transfer |
|
|
O |
|
|
Ar = aryl |
R |
|
+ H2C Ar |
heterolytic |
O |
|
|
|
process |
|
|
|
|
|
R'OH |
|
|
RCOOH |
+ |
R'OCH2Ar |
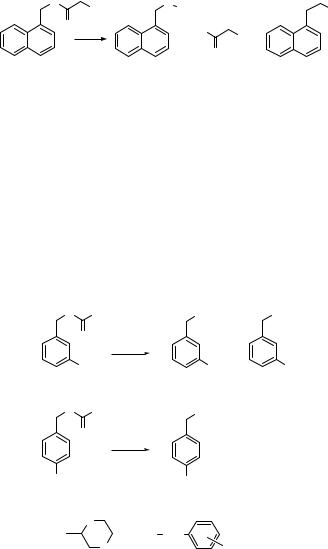
Photolysis of the 1-naphthylmethyl ester of phenylacetic acid (327) in methanol, for example, affords the 1-naphthylmethyl cation–carboxylate anion pair in addition to 1-naphthylmethyl radical–acyloxy radical pair intermediates, which, after decarboxylation, form an adduct with methanol (328, formed along with 329) or an in-cage radical coupling product 330 (Scheme 6.146).1030 The competition between the radical and ionic pathways was found to be very dependent upon the substituents on the naphthalene ring.
O |
Ph |
O Me |
|
Ph |
|
O |
HO |
|
|
|
hν |
Ph + |
+ CO2 |
|
MeOH |
+ |
|
|
O |
|
|
|
|
|
327 |
|
328 |
329 |
330 |
Scheme 6.146
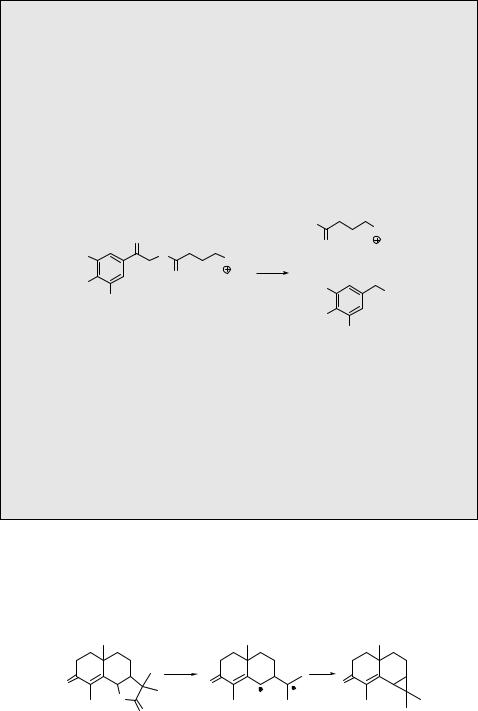
It was discussed in Section 6.2.3 that substituted aromatic compounds in the lowest excited singlet state display behaviour contrasting with those in the ground state, which is related to differing charge distributions in the two electronic states. For example, benzene derivatives substituted with an electron-withdrawing group in the meta position are more
reactive towards photochemical solvolysis than their para-substituted analogues (meta effect).1031–1033 Scheme 6.147 shows the difference in photoreactivity of 3-methoxybenzyl
acetate (meta) (331) and 4-methoxybenzyl acetate (para) (332) in dioxane.327 Irradiation of the meta-derivative leads predominantly (35%) to the product of heterolysis (333), whereas the para-derivative affords products of homolysis (334) only.
O |
CH3 |
OH |
OR |
|
O |
hν |
|
|
|
|
+ |
|
|
|
dioxane |
|
|
OCH |
OCH |
OCH |
331 |
|
3 |
3 |
3 |
|
|
333 |
|
|
|
|
(major) |
(minor) |
O |
CH3 |
OR |
|
|
O |
hν |
|
|
|
|
|
|
|
|
dioxane |
|
|
OCH3 |
|
OCH |
|
|
|
|
3 |
|
332 |
|
|
334 |
|
|
O |
|
|
|
R = |
|
or |
CH2 |
|
|
O |
OCH3 |
334 |
Chemistry of Excited Molecules |
Case Study 6.26: Biology – photoactivatable compounds
Esters of carboxylic acids have been utilized as photoremovable protecting groups (PPGs) (see also Special Topic 6.18) in organic synthesis and biology.1013,1015,1034,1035
For example, the 4-hydroxyphenacyl protecting group has proven to be an efficient tool for analysis of fast biological processes. Its esters are usually water soluble and stable in aqueous solutions in the dark and the photoproducts are nontoxic. The photolysis of the 4-hydroxy-3-methoxyphenacyl ester of g-amino butyric acid (GABA) (335)
(Scheme 6.148) allows for the spatially and temporally controlled rapid release of GABA (336) in CA1 neurons of acute hippocampal brain slices.1036 It has been
demonstrated that the deprotection mechanism proceeds via the excited triplet state (see Case Study 5.3).
|
|
|
HO |
|
|
O |
NH3 |
|
|
O |
MeO |
|
O |
|
336 |
|
|
NH3 |
hν |
HO |
|
O |
+ |
|
MeO |
|
|
|
OMe |
335 |
COOH |
|
|
|
|
HO |
|
|
|
|
|
|
OMe |
|
|
Scheme 6.148 |
Experimental details.1036 CA1 hippocampal neuron from a 7-day-old rat, bathed in a solution of 335 (200 mM), was irradiated by UV flashes from a mercury arc lamp (100 W) coupled to fused-silica optical fibres using transmitting condensers (Figure 3.28).1037 The light-emitting end of the fibre was positioned above the brain slice, producing an approximately 15 25 mm spot. The duration of the light pulse (2 ms) was controlled with an electromechanical shutter. The pulse elicited fast membrane currents due to the specific stimulation of GABA A-receptors (ligand-gated chloride channels) because of the in situ GABA photorelease.
The photodecarboxylation of lactones usually involves the homolytic fission of the C O bond attached to the carbonyl group. Strained ring systems, such as cyclopropane, can be obtained by intramolecular coupling of the resulting biradical intermediate.1025 Scheme 6.149 shows the photodecarboxylation of the a-santonin derivative 337, possessing a g-lactone ring, to give 338 in 56% chemical yield.1038
|
hν |
|
O |
- CO2 O |
O |
|
O |
|
337 |
O |
338 |
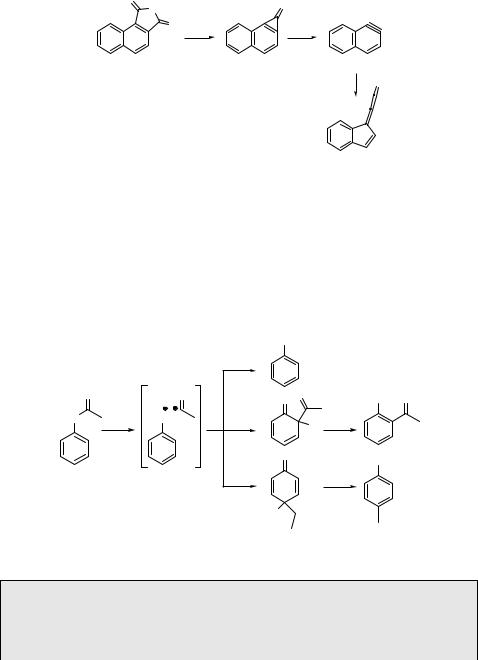
Photolysis of aromatic lactones and anhydrides, such as naphthalene-1,2-dicarboxylic anhydride (339), gives arynes (in this case 340)1039 as reactive intermediates.1040 Matrix isolation spectroscopy (Section 3.10) then allows their study along with other exotic molecules, which are formed as primary or secondary (photo)products (Scheme 6.150).
O |
|
O |
|
O |
|
|
|
|
|
O |
hν |
hν |
|
|
|
|
- CO2 |
- CO |
|
339 |
|
340 |
|
|
|
hν |
O |
|
|
CO |
|
Scheme 6.150
The photo-Fries rearrangement, analogous to the Lewis acid-catalysed (ground state) Fries reaction, is a typical reaction of the aryl esters of carboxylic acids, but also of aryl carbonates, carbamates, sulfonates and other related compounds.1012 Scheme 6.151 shows the photorearrangement of phenyl acetate, which cleaves homolytically at the carbonyl– oxygen single bond from the excited singlet state to give a radical pair. Subsequent hydrogen abstraction by phenyloxy radical from a hydrogen atom donor ([H]) affords phenol, while in-
cage recombination of the radical pair provides 2- or 4-hydroxyacetophenone. It has also been reported that some aryl esters react from their upper triplet states.1012,1041
|
|
|
OH |
|
|
|
|
[H] |
|
|
O |
O |
O O |
OH O |
O |
hν |
O |
|
H |
|
|
|
|
|
|
O |
OH |
|
|
|
|
H  O
O
 O
O
Scheme 6.151
Case Study 6.27: Supercritical CO2 chemistry – photo-Fries reaction
Supercritical solvents, compounds at a temperature and pressure above their thermodynamic critical points, are interesting reaction media because their properties,




 R + COOH
R + COOH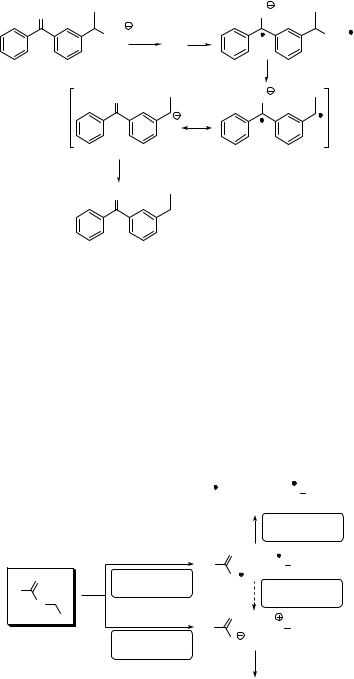





 O
O