
Photochemistry_of_Organic
.pdf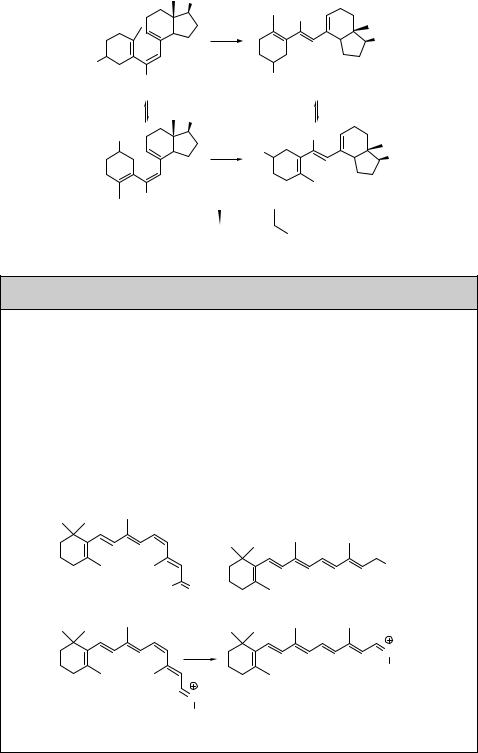
236 |
Chemistry of Excited Molecules |
||
|
|
R |
|
|
|
|
H |
|
|
hν |
R |
|
|
|
|
HO |
|
|
|
H |
|
|
OH |
41a |
|
|
42a |
∆ |
∆ |
R |
∆ ∆ |
|
|
|
|
OH |
|
|
H |
|
|
hν |
HO |
|
|
|
R |
H |
|
|
|
41b |
|
|
42b |
R = 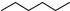
Scheme 6.8
Special Topic 6.1: Vision
The retina of the eye consists of a number of photoreceptor cells containing the visual pigment rhodopsin, a bundle of seven transmembrane helices that enclose the photochemically active chromophore, the imine of 11-cis-retinal (43),574 which is formed by oxidation of the dietary supplement vitamin A (all-trans-retinol, 44; Scheme 6.9). Retinal is covalently linked to the opsin receptor as an iminium salt (45). This system is capable of phototransduction, a process by which light energy is converted into electrochemical potential. The primary photochemical process is E–Z photoisomerization (F 0.65),
which changes the conformation of the opsin molecules and leads to signal transduction cascades.210,575–577 The visual process has an extraordinarily high sensitivity; a retinal rod
registers the absorption of a single photon in a dark-adapted eye, while the response of cones, used for daytime vision, is typically 10–100 times smaller. Enzymes mediate a reverse Z–E isomerization of retinal that subsequently regenerates 11-cis-rhodopsin.
|
|
|
OH |
43 |
H |
O |
44 |
|
|
||
|
|
hν |
NH |
protein
45NH protein
Scheme 6.9
Alkenes and Alkynes |
237 |
Special Topic 6.2: Phototherapy – treatment of hyperbilirubinaemia
Phototherapy or light therapy is exposure to specific wavelengths of visible light to treat health problems, such as acne vulgaris, seasonal and non-seasonal affective disorders or delayed sleep phase syndrome. It is also routinely used in hospitals for treating neonatal jaundice (hyperbilirubinaemia), which is due to an accumulation of the neurotoxic metabolite bilirubin in blood.578 (Z,Z)-Bilirubin (46), a yellow–orange tetrapyrrole derivative, is a product of haem degradation catalysed by haem oxygenase in mammals. This compound is insoluble in water due to intramolecular stabilization by six strong hydrogen bonds (Scheme 6.10); therefore, its direct excretion is impossible. It can be excreted into bile only via a process known as glucuronidation. Formation and excretion are balanced in healthy persons; however, after birth, the high rate of red cell degradation may not be compensated by the initially low liver activity and, as a result, neonatal jaundice occurs. During phototherapy, young patients are exposed to blue or white light to expedite bilirubin elimination. Two major photoprocesses are believed to be involved in the mechanism. The first is the configurational E–Z photoisomerization of 46 to form the 4Z,15 E- isomer 47;579 the second is the less efficient formation of a structural isomer lumirubin (not shown). Because both products are water soluble (the intramolecular hydrogen bonding present in the parent compound is now geometrically suppressed), which permits their direct excretion, both photoreactions help to reduce the bilirubin concentration in blood plasma. It has been found that the initial twisting of the double bond in the excited 46 takes place on a time scale from several hundreds of femtoseconds to a few picoseconds.580
|
|
|
|
O |
O |
|
|
|
N H |
H |
|
|
|
|
H N |
|
|
O |
|
O |
|
H |
|
|
O |
|
O |
|
|
H N |
|
hν |
H N |
|
|
|
|
|
|
N |
|
|
N |
|
H |
O |
|
H |
O |
|
|
|
||
N H |
O |
|
N H |
O |
O |
H |
|
O |
H |
|
|
|
||
46 |
|
|
47 |
|
Scheme 6.10
Photosensitized Isomerization
Sensitization is often indispensable for inducing reaction from the triplet state and it is practical in the case of aliphatic non-conjugated alkenes, which absorb weakly over 200 nm. The triplet energy (ET) of the sensitizer should be higher than that of the acceptor to afford efficient energy transfer (Section 2.2.2). Nevertheless, triplet
238 |
Chemistry of Excited Molecules |
sensitizers such as toluene (ET ¼ 346 kJ mol 1) and p-xylene (ET ¼ 337 kJ mol 1) are known to induce endothermic isomerization of aliphatic alkenes, such as (E)-3,4- dimethylpent-2-ene, the ET of which is higher than 345 kJ mol 1 (Table 6.3).581 When ET of the sensitizer is too low, the isomerization is inefficient or absent. The dependence of PSS on ET is interpreted in terms of a selective, energetically more efficient energy transfer to one of the ground-state isomers – the thermodynamically less stable Z-isomer.
Table 6.3 Sensitized E–Z isomerization of (E)-3,4-dimethylpent-2-ene
Sensitizer |
ET/ kJ mol 1 |
[E]:[Z]a |
Benzene |
353 |
61:39 |
p-Xylene |
337 |
66:34 |
Pentamethylbenzene |
333 |
—b |
aPSS concentration ratio.581
bPSS has not been reached after extended irradiation.
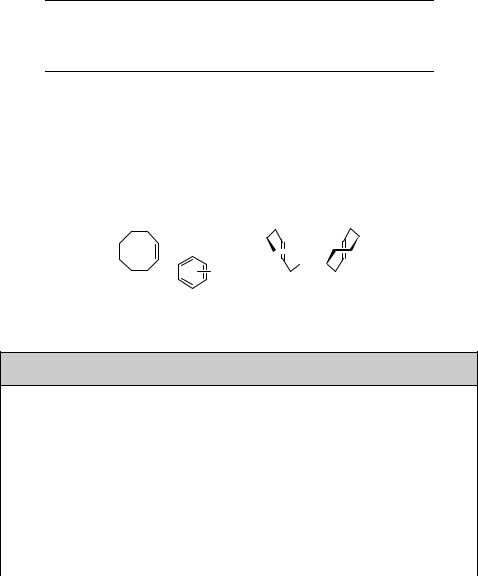
Irradiation of (Z)-cyclooctene in the presence of a triplet sensitizer, such as xylene (ET 337 kJ mol 1), affords the E-isomer, although in a very low chemical yield.582 In
contrast, the singlet state sensitized isomerization by benzene polycarboxylate derivatives (ES 420 kJ mol 1) leads to (E)-cyclooctene in up to 26% chemical yield (Scheme 6.11).583
hν

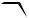 + (COOR)x
+ (COOR)x
Scheme 6.11
Special Topic 6.3: Asymmetric photochemical synthesis
Asymmetric synthesis introduces one or more new features of chirality in a molecule. Several approaches are possible. In general, preferential formation of an enantiomer or diastereoisomer is achieved as a result of the influence of a chiral element present in the substrate, a reagent, catalyst or the environment. Chirality control is also possible in the electronically excited state,584 as demonstrated in the following examples.
Excitation with circularly polarized light (CPL) is an interesting direct method of asymmetric synthesis, which is based on preferential excitation of one of the enantiomers thanks to a difference in the molar absorption coefficients towards left or right CPL at a given wavelength.585 Unfortunately, only a small enantiomeric excess (ee) can be generated by this method. Some scientists, nevertheless, believe that

Alkenes and Alkynes |
239 |
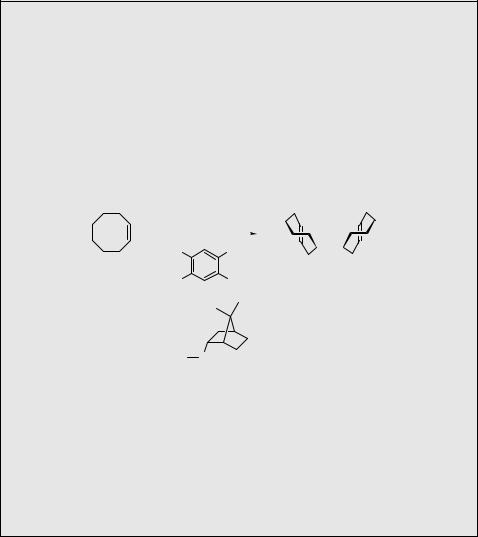
asymmetric induction by CPL may have sparked the abiotic synthesis of optically active molecules in nature. For example, chiral induction can enhance the concentration of one enantiomer by photochemical deracemization of sterically crowded ethenes (Scheme 6.12; ee ¼ 0.07%),586 or nonracemic 6p-electrocyclization (see also Scheme 6.23) of 48 to 49, followed by oxidation to hexahelicene (Scheme 6.13; ee was below 0.5%).587
O O
left-CPL right-CPL
S S
Scheme 6.12
|
H |
CPL |
I2, O2 |
|
H |
48 |
49 |
Scheme 6.13
Enantiodifferentiating photosensitization involves a prochiral or racemic substrate interacting with an optically active sensitizer via quenching (Section 2.2.2) or exciplex formation (Section 2.2.3),585 rather than in the subsequent thermal process. Asymmetric photosensitization requires only a catalytic amount of the chiral sensitizer. An example is provided in Case Study 6.2 (E–Z photoisomerization).
Achiral molecules can crystallize in a chiral space group or form inclusion crystals
of achiral guest and chiral host molecules in the absence of any external source of chirality.588–590 Irradiation of such solid-state samples may then generate optically pure
compounds in high chemical yields. This type of asymmetric induction is introduced later in Special Topic 6.5 and Case Study 6.21.
Many other methods of asymmetric induction in photochemistry, such as templateinduced enantioselective photoreactions,591asymmetric photochemistry in zeolites,592 or simple photoreactions of a chiral starting material, parallel their ground-state chemistry counterparts. Diastereoselective photocycloaddition in the Paterno`–B€uchi reaction of chiral reactants (Case Study 6.17) may serve as an additional example.
240 |
Chemistry of Excited Molecules |
Case Study 6.2: Asymmetric synthesis – enantiodifferentiating photoisomerization
Both singlet and triplet photosensitization of (Z)-cyclooctene (50) produces the (E)- cyclooctene enantiomers [( )-(R)-51 and ( þ )-(S)-51; Scheme 6.14]. High enantiodifferentiation (Special Topic 6.1) has been achieved with singlet excited chiral
polyalkyl benzenepolycarboxylates, whereas triplet sensitization (e.g. with chiral alkyl benzyl ethers) gives low optical yields only.583,593 For example, when a chiral tetra[( )-
bornyl]oxycarbonyl singlet sensitizer 52 was used at 88 C, ( )-(R)-51 was obtained in 9% chemical yield and 41% optical purity at 12% conversion. The effect is attributed to specific interactions within an exciplex formed between sensitizer and alkene.
|
|
hν |
|
+ |
|
|
|
|
|
|
ROOC |
|
COOR |
|
50 |
|
|
|
(-)-(R)-51 (+)-(S)-51 |
|
ROOC |
|
|
|
|
52 |
COOR |
||
|
|
|
|
|
R = CH2
Scheme 6.14
Experimental details.583 A pentane solution (200–300 ml) of 50 (200 mM) and the sensitizer 52 (5 mM) was irradiated in a quartz vessel with a low-pressure mercury lamp (30 W) through a Vycor sleeve (>250 nm) (Figure 3.9) under an argon atmosphere at88 C for 72 h. After irradiation, the product was extracted from the solution with aqueous silver nitrate at 0 C and the combined aqueous extracts were washed with pentane and then added dropwise into concentrated ammonia solution to liberate 51, which was subsequently extracted with pentane.
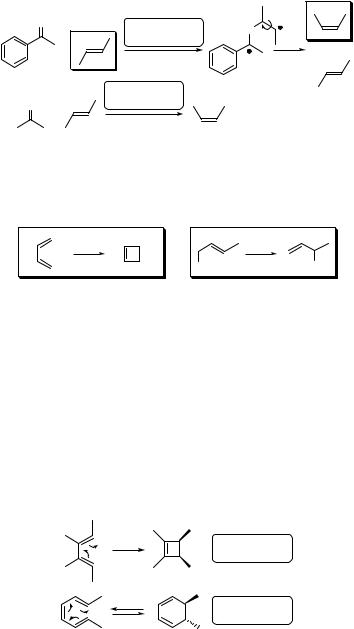
An alternative mechanism for photosensitized isomerization, termed the Schenck mechanism,594 involves the formation of an adduct between an alkene and a triplet sensitizer [a Paterno`–B€uchi-like (Section 6.3.2) biradical], in which rotation about the central bond is fast relative to bond breaking. The corresponding alkenes formed after elimination of the sensitizer molecule are in a concentration ratio close to that obtained by the thermal process. This mechanism was thought to be involved when the triplet energy transfer is endergonic (i.e. not efficient), as is the case with the sensitization of an alkene (ET > 325 kJ mol 1) by an excited acetophenone (ET ¼ 310 kJ mol 1) (Scheme 6.15).595 In contrast, a conventional photosensitized E–Z isomerization mechanism dominates in the presence of acetone (ET ¼ 332 kJ mol 1), which transfers energy exergonically.
|
Alkenes and Alkynes |
241 |
3O* |
Schenck |
O |
|
mechanism |
|
|
|
|
|
+ |
+ |
|
triplet |
|
3O* |
sensitization |
|
|
+ |
|
Scheme 6.15
6.1.2Alkenes: Electrocyclic and Sigmatropic Photorearrangement
R R
Recommended review articles.487,523,528,530,532,564,596–602
Selected theoretical and computational photochemistry references.16,480,534,535,538,603–610
Electrocyclic Photorearrangements
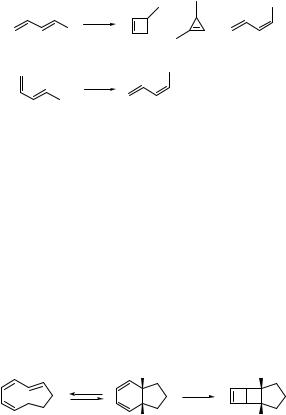
Electrocyclic ring-opening and ring-closure reactions are unimolecular pericyclic processes which involve a change of the p- and s-bond positions within a conjugated system in a cyclic transition state. According to the Woodward–Hoffmann orbital symmetry rules,336 the 4n electron systems in the excited state react through a disrotatory mode, whereas those with 4n þ 2 electrons react through a conrotatory pathway (Section 4.9; Scheme 6.16). Such a stereospecific process is known to be the opposite of that observed for reactions initiated thermally.
hν |
4π−disrotatory |
|
ring closure |
hν |
6π−conrotatory |
hν |
processes |
|
Scheme 6.16
Only the singlet state interconversions of conjugated dienes and cyclobutenes are taken into consideration here, because the triplet state is generally not involved in this process.596 There are two important factors that affect electrocyclization: (1) reaction
242 |
Chemistry of Excited Molecules |
efficiency is usually lowered by a concurrent E–Z isomerization (Section 6.1.1) along the double bond and (2) s-cis-diene conformation must be achieved before cyclization proceeds. Direct irradiation of dienes may result in wavelength-dependent photoproduct formation, which is related to different absorption properties of the diene conformers. For example, (E)-penta-1,3-diene (53), irradiated at 254 nm, gives 3-methylcyclobut-1-ene (54) in a low quantum yield (F ¼ 0.03) and two other products; however, when 53 is irradiated at 229 nm, where the s-trans-conformation absorbs predominantly, mostly E–Z isomerization is observed (Scheme 6.17).611
|
254 nm |
+ |
|
+ |
|
s-trans-53 |
54 |
|
229 nm
(only)
s-cis-53
Scheme 6.17
In another example, cis-bicyclo[4.3.0]nona-2,4-diene (55) undergoes either the intramolecular 4p-ring closure or 6p-ring opening depending upon the wavelength of excitation (Scheme 6.18).612 Irradiation at 254 nm results in a 7:3 bicyclononadiene 55–cyclonona- triene 56 photostationary mixture, reflecting the molar absorption coefficient ratio («25455 ¼ 3800 M 1 cm 1; «25456 ¼ 1000 M 1 cm 1) to some extent, while the production of a small amount of the tricyclononene 57 is observed at 300 nm. Since the triene 56 absorbs considerably at this wavelength («30056 ¼ 2000 M 1 cm 1; «30055 ¼ 50 M 1 cm 1), the photostationary ratio shifts in favour of 55, thus promoting non-efficient production of 57.
H |
H |
254 nm |
300 nm |
H |
H |
5655 57
Scheme 6.18
Apart from photocyclization reactions, simple trienes undergo many types of other phototransformations, including E–Z photoisomerization (Section 6.1.1), sigmatropic shifts (see later) or photoadditions (Section 6.1.4). For example, conformational control and possibly equilibration between the excited conformers result in a
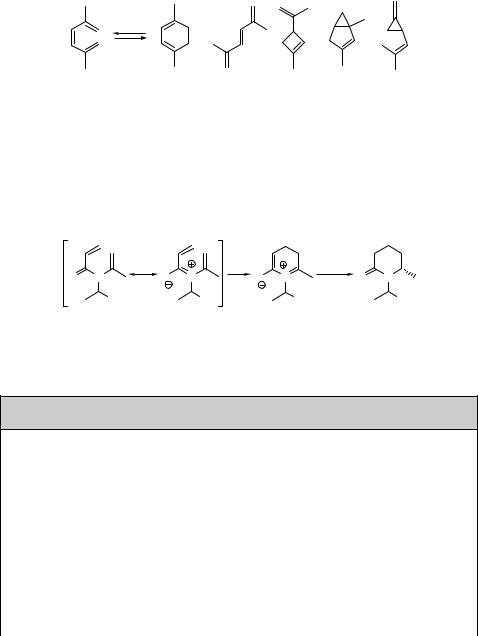
Alkenes and Alkynes |
243 |
complicated mixture of photoproducts obtained upon irradiation of 2,5-dimethylhex- atriene (58) at 313 nm, although 6p photocyclization to 59 is still the major reaction pathway (Scheme 6.19).613
|
hν |
+ |
+ |
+ |
+ |
|
hν |
||||
|
|
|
|
|
|
58 |
|
59 |
|
|
|
Scheme 6.19
Scheme 6.20 shows an example of photochemical electrocyclization of the enantiomerically pure acrylamide 60 in the presence of NaBH4, which reduces the
immonium functionality in the primary product 61 to give the lactam 62 in 75% chemical yield.614
O N |
hν |
O N |
NaBH4 |
O N |
O N |
||
Ph |
Ph |
Ph |
Ph |
|
60 |
61 |
62 |
Scheme 6.20
Special Topic 6.4: Photoproduction of vitamin D
Vitamin D is associated with biological functions, such as bone formation, immune system responses, cell defences and anti-tumour activity.615,616 Vitamin D comes in
two closely related forms, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), and their metabolites. Both vitamin D2 and D3 occur naturally in some foods. However, vitamin D3 (63) can also be synthesized in skin cells called keratinocytes from 7-dehydrocholesterol (provitamin D; 64), which undergoes a photochemical sixelectron conrotatory electrocyclic ring opening at 280 nm to previtamin D3 (41; see also Scheme 6.8), which spontaneously isomerizes to 63 in a thermal antarafacial hydride [1,7]-sigmatropic shift (Scheme 6.21). Both vitamin D2 and D3 are subsequently converted to active hormone 1,25-D by enzymes in several steps. The recommended daily intake of vitamin D for humans is 5–10 mg per day. For example, 15 ml of fish liver oils and 100 g of cooked salmon contain approximately 35 and 10 mg
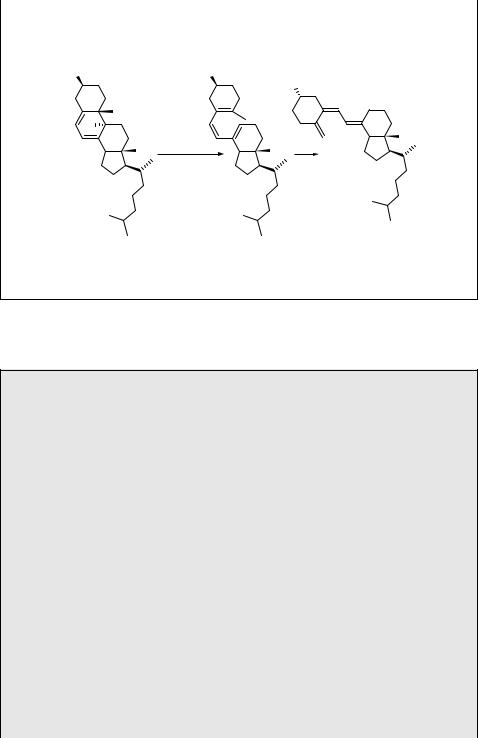
244 |
Chemistry of Excited Molecules |
of vitamin D, respectively, and full-body summer exposure to sunshine at midday for 15–20 min produces 250 mg of vitamin D.
HO |
HO |
HO |
|
|
|
|
H |
|
|
hν |
∆ |
|
keratinocytes |
|
64 |
41 |
63 |
Scheme 6.21
Case Study 6.3: Mechanistic photochemistry – previtamin D photochemistry
The photochemistry of vitamin D (see also Special Topic 6.4 above and Scheme 6.8)
has also played a central role in the development of modern organic photochemistry.564,598,617,618 The concept of non-equilibration of excited rotamers (NEER;
Section 6.1.1) has been used to explain the excitation-wavelength dependence of E–Z isomerization (Section 6.1.1) of previtamin D3 (41).619 Whereas the quantum yield for E–Z isomerization decreases with increasing wavelength, the formation efficiencies of the 6p-electron conrotatory ring-closure products, diastereomeric 7-dehydrocholester- ol (provitamin D) (64) and lumisterol (65) (Scheme 6.22), increase dramatically. This was found to occur on the basis of a participation of both the S1 and S2 excited states in the photoreaction.620 For example, the quantum yields of 64 and 65 formation were: F64 ¼ 0.01 and F65 ¼ 0.05 at 285 nm, but F64 ¼ 0.08 and F65 ¼ 0.21 at 325 nm. Vitamin D3 is naturally synthesized in human skin by a spontaneous thermal antarafacial hydrogen [1,7]-sigmatropic shift in previtamin D3, which is produced from 64 upon solar UVB irradiation in the first step.621
Experimental details.620 Sample solutions of previtamin D3 (2 10 3 M) in a 1 cm pathlength UV cell were purged with nitrogen and irradiated using a pulsed UV dye laser at 300 nm (Special Topic 3.1), containing Rhodamine 6G as a sensitizer in methanol or a methanol–water mixture (1:1, v/v). After irradiation, an aliquot of the sample solution was analysed by HPLC.

|
Alkenes and Alkynes |
|
245 |
HO |
HO |
|
HO |
H |
hν |
hν |
H |
|
hν |
hν |
|
64 |
41 |
65 |
Scheme 6.22
(Z)-Diarylethenes, such as stilbene, also undergo a 6p-electrocyclization reaction upon irradiation from the singlet p,p state, but not upon triplet sensitization.487,529,599,622 The
photochemical orbital symmetry-allowed conrotatory closure leads to the trans-dihydro derivative 66 (Scheme 6.23), which can subsequently be oxidized by oxygen or iodine to the corresponding arene (phenanthrene) in nearly quantitative yield.561
hν |
hν |
H |
I2 or O2 |
hν |
∆ |
H |
|
|
|
||
|
|
|
66 |
E-Z |
6π−conrotatory |
oxidation |
|
isomerization |
photoprocess |
|
|
|
|
||
Scheme 6.23
This reaction has been exploited as a convenient way of producing helicene derivatives (see also Special Topic 6.3).487,599,622 [7]Helicene (67) is, for example,
produced from 1,4-bis[(E)-2-(naphthalen-2-yl)ethenyl]benzene (68) in 14% overall chemical yield (Scheme 6.24).623
1. hν
2. oxidation
68 |
67 |
Scheme 6.24
