
- •VOLUME 2
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •CARBON.
- •CARDIAC CATHETERIZATION.
- •CARDIAC LIFE SUPPORT.
- •CARDIAC OUTPUT, FICK TECHNIQUE FOR
- •CARDIAC OUTPUT, INDICATOR DILUTION MEASUREMENT OF
- •CARDIAC PACEMAKER.
- •CARDIAC OUTPUT, THERMODILUTION MEASUREMENT OF
- •CARDIOPULMONARY BYPASS.
- •CARDIOPULMONARY RESUSCITATION
- •CARTILAGE AND MENISCUS, PROPERTIES OF
- •CATARACT EXTRACTION.
- •CELL COUNTER, BLOOD
- •CELLULAR IMAGING
- •CEREBROSPINAL FLUID.
- •CHEMICAL ANALYZERS.
- •CHEMICAL SHIFT IMAGING.
- •CHROMATOGRAPHY
- •CO2 ELECTRODES
- •COBALT-60 UNITS FOR RADIOTHERAPY
- •COCHLEAR PROSTHESES
- •CODES AND REGULATIONS: MEDICAL DEVICES
- •CODES AND REGULATIONS: RADIATION
- •COGNITIVE REHABILITATION.
- •COLORIMETRY
- •COMPUTERS IN CARDIOGRAPHY.
- •COLPOSCOPY
- •COMMUNICATION AIDS FOR THE BLIND.
- •COMMUNICATION DEVICES
- •COMMUNICATION DISORDERS, COMPUTER APPLICATIONS FOR
- •COMPOSITES, RESIN-BASED.
- •COMPUTED RADIOGRAPHY.
- •COMPUTED TOMOGRAPHY
- •COMPUTED TOMOGRAPHY SCREENING
- •COMPUTED TOMOGRAPHY SIMULATOR
- •COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION
- •COMPUTER-ASSISTED DETECTION AND DIAGNOSIS
- •COMPUTERS IN CARDIOGRAPHY.
- •COMPUTERS IN THE BIOMEDICAL LABORATORY
- •COMPUTERS IN MEDICAL EDUCATION.
- •COMPUTERS IN MEDICAL RECORDS.
- •COMPUTERS IN NUCLEAR MEDICINE.
- •CONFOCAL MICROSCOPY.
- •CONFORMAL RADIOTHERAPY.
- •CONTACT LENSES
- •CONTINUOUS POSITIVE AIRWAY PRESSURE
- •CONTRACEPTIVE DEVICES
- •CORONARY ANGIOPLASTY AND GUIDEWIRE DIAGNOSTICS
- •CRYOSURGERY
- •CRYOTHERAPY.
- •CT SCAN.
- •CUTANEOUS BLOOD FLOW, DOPPLER MEASUREMENT OF
- •CYSTIC FIBROSIS SWEAT TEST
- •CYTOLOGY, AUTOMATED
- •DECAY, RADIOACTIVE.
- •DECOMPRESSION SICKNESS, TREATMENT.
- •DEFIBRILLATORS
- •DENTISTRY, BIOMATERIALS FOR.
- •DIATHERMY, SURGICAL.
- •DIFFERENTIAL COUNTS, AUTOMATED
- •DIFFERENTIAL TRANSFORMERS.
- •DIGITAL ANGIOGRAPHY
- •DIVING PHYSIOLOGY.
- •DNA SEQUENCING
- •DOPPLER ECHOCARDIOGRAPHY.
- •DOPPLER ULTRASOUND.
- •DOPPLER VELOCIMETRY.
- •DOSIMETRY, RADIOPHARMACEUTICAL.
- •DRUG DELIVERY SYSTEMS
- •DRUG INFUSION SYSTEMS
28.Stein J, Bortfeld T, Dorschel B, Schlegel W. Dynamic X-ray compensation for conformal radiotherapy by means of multileaf collimation. Radiother Oncol 1994;32:163–173.
29.Boyer AL, Yu CX. Intensity-modulated radiation therapy with dynamic multi-leaf collimators. Semin Radiat Oncol 1999;32:48–59.
30.Nutting C, Dearnaley DP, Webb S. Intensity modulated radiation therapy: a clinical review. Br J Radiol 2000;73:459–469.
31.Intensity Modulated Radiation Therapy Collaborative Working Group, Intensity-modulated radiotherapy: current status and issues of interest. Int J Radiat Oncol Biol Phys 2001; 51:880–914.
32.Purdy JA. Advances in three-dimensional treatment planning and conformal dose delivery. Semin Oncol 1997;24:655–672.
33.Boyer AL, Xiang L, Xia P. Beam shaping and intensity modulation in modern technology of radiation oncology. Van Dyk J, editors. Modern Technology of Radiation Oncology. Medical Physics Publishing; Madison (WI): 1999.
34.Wang X, et al. Dosimetric verification of intensity-modulated fields. Med Phys 1996;23:317–327.
35.Xing L, et al. Dosimetric verification of a commercial inverse treatment planning system. Phys Med Biol 1999;44:463–478.
36.Mackie TR, et al. Tomotherapy: a new concept for the delivery of conformal radiotherapy. Med Phys 1993;20:1709–1719.
37.Yu CX. Intensity-modulated arc therapy with Dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol 1995;40:1435–1449.
38.Mackie TR, et al. Tomotherapy. Semin Radiat Oncol 1999; 9:108–117.
39.Svenssen R, Lind BK, Brahme A. A new compact treatment unit design combining narrow pencil beam scanning and segmental multileaf collimation. Radiothr Oncol 1999;51:S21.
40.Schweikard A, Tombropoulos R, Adler JR. Robotic radiosurgery with beams of adaptable shape. In: Ayache N, editor. Computer Vision and Robotics in Medicine. Springer-Verlag; Berlin (Heidelberg): 1995.
41.Webb S. Conformal intensity-modulated radiotherapy (IMRT) delivered by robotic linacs—testing IMRT to the limit? Phys Med Biol 1999;44:1639–1654.
42.Woo SY, et al. A comparison of intensity modulated conformal therapy with a conventional external beam steoreotactic radiosurgery system for the treatment of single and multiple intracranial lesions. Int J Radia Oncol Biol Phys 1996; 35:593–597.
43.Kramer BA, et al. Dosmetric comparison of stereotactic radiosurgery to intensity modulated radiotherapy. Radia Oncol Invest 1998;6:18–25.
44.Cardinale RM, et al. A comparison of three stereotactic radiosurgery techniques: arcs vs non-coplanar fixed fields vs intensity modulation. Int J Radia Oncol Bio Phys 1998;42: 431–436.
45.Botfeld T. Optimized planning using physical objects and constraints. Semin Radiat Oncol 1999;9:20–34.
46.Chvetsov AV, Calvetti D, Sohn JW, Kinsella T. Regularization of inverse planning for intensity-modulated radiotherapy. Med Phys 2005;32:501–514.
47.Sauer OA, Shepard DM, Mackie TR. Application of constrained optimization to radiotherapy planning. Med Phys 1999;26:2359–2366.
48.Spirou SV, Chui CS. A gradient inverse planning algorithm with dose-volume constraints. Med Phys 1998;25:321–333.
49.Hristov DH, Stavrev P, Sham E, Fallone BG. On the implementation of dose-volume objectives in gradient algorithms for inverse treatment planning. Med Phys 2002;29:848–856.
50.Wu Q, Mohan R, Niemierko A, Schmidt-Ullrich R. Optimization of intensity-modulated radiotherapy plans based on the equivalent uniform dose. [comment]. Int J Radia Oncol Biol Phys 2002;52:224–235.
COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION |
277 |
51.Das S, et al. Beam orientation selection for intensity-modulated radiation therapy based on target equivalent uniform dose maximization. Int J Radia Oncol Biol Phys 2003;55:215–224.
52.Choi B, Deasy JO. The generalized equivalent uniform dose function as a basis for intensity-modulated treatment planning. Phys Med Biol 2002;47:3579–35894.
53.Wang XH, et al. Optimization of intensity 3D conformal treatment plans based on biological indices. [comment]. Radiothre Oncol 1995;37:140–152.
54.Baydush AH, Marks LB, Das SK. Penalized likelihood fluence optimization with evolutionary components for intensity modulated radiation therapy treatment planning. Med Phys 2004;31:2335–2343.
55.Webb S. Optimization by simulated annealing of threedimensional conformal treatment planning for radiation fields defined by a multileaf collimator. Phys Med Biol 1991;36:1201– 1226.
56.Hristov DH, Fallone BG. An active set algorithm for treatment planning optimization. Med Phys 1997;24:1455–1464.
57.Ezzell GA. Genetic and geometric optimization of threedimensional radiation therapy treatment planning. Med Phys 1996;23:293–305.
58.Olivera GH, et al. Maximum Likelihood as a common computational framework in tomotherapy. Phys Med Biol 1998;43: 3277–3294.
59.Llacer J, Solberg TD, Promberger C. Comparative behavior of the dynamically penalized likelihood algorithm in inverse radiation therapy planning. Phys Med Biol 2001;46:2637–2663.
60.Yan H, Yin F, Guan H, Kim JH. Fuzzy logic guided inverse treatment planning. Med Phys 2003;30:2675–2685.
61.McKezie AL. How should breathing motion be combined with other errors when drawing margins around clinical target volumes? Br J Radiol 2000;73:973–977.
62.Bergstrom P, Lofroth PO, Widmark A. High precision conformal radiotherapy (HPCRT) of prostate cancer—a new technique for exact positioning of the prostate at the time of treatment. Int J Radiat Oncol Biol Phys 1998;42:305–311.
63.The BS, et al. Intensity modulated radiation therapy (IMRT) following prostateectomy: more favorable acute genitourinary toxicity profile compared to primary IMRT for prostate cancer. Int J Radiat Oncol Biol Phys 2001;49:465–472.
64.Matsinos E. Current status of the CBCT project at Varian. Proc SPIE 2005.
65.Colbeth RE, Roos PG, Mollov IP. Flat panel CT detector for sub-second volumetric scanning. Proc SPIE 2005.
See also PHANTOM MATERIALS IN RADIOLOGY; RADIATION THERAPY SIMULATOR.
COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION
FREDERIC H. FAHEY
Children’s Hospital Boston
HARVEY A. ZIESSMAN
Johns Hopkins University
INTRODUCTION
Single photon emission computed tomography (or SPECT) provides a three-dimensional (3D) representation of the distribution within a patient’s body of a radiopharmaceutical that was given as part of a diagnostic nuclear
278 COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION
medicine study. Diagnostic nuclear medicine provides a unique way of imaging physiology and function. One can administer to a patient a pharmaceutical with a radioactive marker, and then use external detectors to determine where that ‘‘radiopharmaceutical’’ has distributed within the patient’s body. The distribution of the radiopharmaceutical in the body depends on its specific biology. For example, suppose a patient is given a small amount of radioactive iodine (e.g., iodine-131 or 131I). Since the thyroid naturally metabolizes iodine, some portion of the radioactive iodine will be preferentially incorporated into the thyroid with the rest going to other organs with lower concentrations. The amount of 131I that will go to the thyroid will depend on whether the thyroid is functioning in a normal, hyperactive, or hypoactive fashion. By acquiring an image from this patient, one can determine whether certain regions of the thyroid are more active than others. For example, there may be hyperactive nodules within the thyroid. In this manner, nuclear medicine provides a unique opportunity to view the patient’s physiology and not just the anatomy. Devices known as gamma cameras are used to provide images of the in vivo distribution (i.e., the distribution within the body) of the radiopharmaceutical. From these image data, the patient’s specific physiology can be inferred.
The images produced by gamma cameras are two dimensional (2D) representations of a 3D object. In some cases, this is adequate to interpret the study. However, in many cases, the ambiguity introduced by activity in the overlying and underlying tissue can make it very difficult to infer the in vivo distribution of the radiopharmaceutical appropriately. In these cases, a 3D representation is necessary. Single photon emission computed tomography provides such a 3D representation. For example, SPECT can make it easier to determine whether the activity is reduced in the basal or apical aspect of the inferior wall of the myocardium or whether the activity in a tumor seen in the chest is in a rib or in the lung. As will be discussed, the 3D SPECT images are generated by a computer from a series of images taken about the patient at different angles. Since the photons used to generate the images are emitted from within the patient’s body, SPECT is considered emission computed tomography, which is in contrast to transmission computed tomography (CT) where the X-ray photons emanate from an X-ray tube and are transmitted through the patient. In the early development of SPECT, the word ‘‘single’’ was used to distinguish SPECT from positron emission tomography (PET), which uses two photons to localize each event and requires different instrumentation. For these reasons, the generation of a 3D representation of the in vivo distribution of radiopharmaceutical that is not a positron emitter is referred to as single photon emission computed tomography or SPECT.
In 1963, a method of nuclear tomographic imaging was developed by Kuhl and Edwards (1). This method used a specially designed scanning device along with a processing method called simple backprojection to generate its 3D images. This early research in SPECT predates Hounsfield’s work in CT by 10 years. Kuhl and Edwards (2) subsequently developed a dedicated SPECT device for
imaging the brain known as the Mark IV scanner. During the 1970s, several devices were developed that were dedicated to SPECT, specifically for brain imaging. However, techniques were also being investigated to use the gamma camera that was used for all other nuclear imaging applications for SPECT as well. This required the gamma camera to rotate around the patient’s body in order to acquire different angular views. Keyes et al. (3) developed the first prototype of the rotating gamma camera by mounting a standard gamma camera head to the gantry of a decommissioned cesium-137 radiation therapy unit. At about the same time, Jaszczak et al. (4) developed a commercial version of the rotating gamma camera. Although the development of dedicated SPECT devices continues, by far the majority of devices used clinically are rotating gamma cameras. For this reason, this chapter will devote most of its attention to the use of the rotating gamma camera.
SPECT DATA ACQUISITION AND PROCESSING
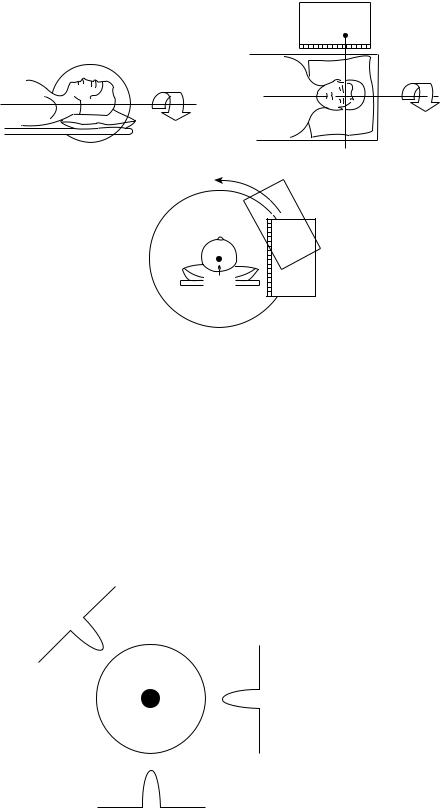
For SPECT data acquisition, a series of images are obtained at a number of different viewing angles. Typically, these images are acquired in a circular arc about the patient with the axis of rotation parallel to the long axis of the patient’s body. This acquisition geometry is shown in Fig. 1. The images acquired at each viewing angle are referred to as ‘‘projection images’’ or ‘‘projections’’. These projections are acquired at a number of evenly spaced viewing angles over either a 360 or a 1808 arc. There is a minimum number of projections that will assure adequate angular sampling at the periphery of the object being imaged which will lead to high quality, tomographic images. For SPECT, this number is between 50 and 150, depending on the size of the object and the spatial resolution of the system. The gamma camera is usually equipped with a multihole, parallel-hole collimator that assures that the photons interacting in the radiation detector of the gamma camera traveled from the patient to the detector on a ray that is perpendicular to the detector surface. Thus, if one knows where the photon interacted in the detector, one can assume that the photon was emitted from a point that was along this ray. In Fig. 1, we refer to this ray as the ‘‘line of origin’’.
Consider a small, high contrast feature in the center of a cylindrical object that contains some radioactivity as shown in Fig. 2. We can acquire some number of projections (3 in the example shown in Fig. 2). If we consider the tomographic plane passing through the center of the tumor, each of the projections will look reasonably similar with a single intensity corresponding to the tumor. The process by which we generate a tomographic image from this series of projections is referred to as ‘‘tomographic reconstruction’’. We can generate a tomographic image of this simple object by filtering and adding the projections, each oriented at the angle associated with that specific projection. This reconstruction technique is referred to as ‘‘filtered backprojection’’. In many cases, additional smoothing is applied using a windowing filter that can control the sharpness and noise associated with
COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION |
279 |
Axis of
Axis of |
|
rotation |
rotation |
|
|
|
|
|
|
line of |
Figure 1. The geometry for SPECT |
|
origin |
|
|
data acquisition with a rotating |
|
|
|
|
|
|
gamma camera is shown. The |
|
|
camera rotates acquiring projection |
|
|
images at different angles about the |
|
|
patient. These data are subsequently |
|
|
reconstructed into cross-sectional |
|
|
slices that indicate the 3D in vivo |
|
|
distribution of the radiopharmaceu- |
Axis of |
|
tical within the patient. (Reprinted |
rotation |
|
from Henkin R., editor, Nuclear |
|
|
Medicine. P 235, Copyright # 1996 |
|
|
with permission from Elsevier.) |
the reconstructed image. The windowing functions that are typically used include the Hanning, Hamming, SheppLogan, and Butterworth filters. Depending on the signal and noise content of the underlying projection data, one can choose an appropriate windowing filter for the best image quality. Figures 3a–c shows three images that are filtered backprojection reconstructions of the same raw data (in this case, a brain study) using three different windowing filters (sharp filter, moderate filter and smooth filter, respectively). One can note the differences in image quality that one can attain by simply varying the filtering.
In addition, different clinical applications (e.g., brain vs. cardiac SPECT) may require different reconstruction filters. Therefore, it is very important to select the most
Projection |
3 |
|
Projection 2
Projection 1
Figure 2. The object in the middle has a central region of high intensity. Three projections about the object are also shown. In a typical SPECT study, 50–150 projections are acquired about the object over 180 or 3608.
appropriate reconstruction filter for each clinical application of SPECT.
Although filtered backprojection has traditionally been the most common means of SPECT reconstruction, iterative reconstruction methods are currently available on newer SPECT systems. These methods utilize a ‘‘feedback’’ approach to generate the tomographic data. These methods start with an initial estimate of the object. This estimate may assume the object is totally uniform or it may use a filtered backprojection reconstruction of the object. From this estimate, a series of projections are calculated along the same viewing angles as the ‘‘real’’ projections, that is the acquired, raw data. If the estimate is close to the true object, then the calculated projections would be very similar to the real projections. If they are not similar, then the variations between the two are determined (either as a ratio or a difference) and used to alter the initial estimate. The process is then repeated. A new set of calculated projections are generated and again compared to the real projections. Presumably, each iteration provides a better estimate of the true object. In other words, the estimates should ‘‘converge’’ to a good representation of the true object. Typically, some statistic such as the ‘‘likelihood’’ or the ‘‘entropy’’ is used to determine how well the method is converging or when to stop the iterative process. In many cases, it can take tens or even hundreds of iterations before the reconstructed estimate converges. Thus, these methods have traditionally been quite slow, much slower than filtered backprojection. However, an advantage of these methods is the ability to take into account the physics of data collection and the statistics of the noise in the images to provide a more accurate reconstruction. Another advantage is that these methods are not as susceptible to some of the artifacts that one encounters in filtered backprojection. Much of the research in this area has centered on the development of methods that are more efficient or converge more

280 COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION
Figure 3. The SPECT brain study with four different reconstructions. Figures 3a–c are reconstructed with three different filters (smooth, moderate and sharp filter, respectively). Figure 3d is reconstructed with an iterative method known as OSEM.
quickly. With the increasing speed of computers and the development of more efficient iterative algorithms, the clinical application of these methods has become feasible and many newer SPECT systems provide iterative reconstruction methods as an option. Figure 3 shows a comparison with the same raw SPECT data reconstructed with both an iterative method (3d) and filtered backprojection (3a–c).
Consider a radiopharmaceutical that basically distributes uniformly within the body. Those photons that are emitted from deep within the body will have to travel through more tissue to reach the gamma camera than those emitted on the periphery of the body. In turn, those photons that must traverse more tissue are more likely to be absorbed within the body and, therefore, are less likely to be detected than those emitted at the periphery. In other words, the signal from deep-seated tissues is ‘‘attenuated’’ as compared to the surface tissues due to self-absorption of the photons within the object. To measure the amount of activity at different locations within the object accurately, one must apply a correction for photon attenuation. There are two basic approaches to attenuation correction in SPECT, one that assumes that the attenuation is uniform within the object, and one that considers the fact that different tissues may have varying absorption characteristics making the attenuation nonuniform.
In much of the body, the composition and density of the soft tissue is basically constant and thus one can assume a single attenuation property for all of the tissue. This assumption is apt, for example, in brain imaging. To apply uniform attenuation correction, one need only know where the body outline is located relative to where the radiopharmaceutical has distributed. For a particular point of interest within the object, the mean distance to the body outline is estimated and, using this estimate, the expected amount of photon attenuation from that point is determined. By multiplying the amount of radioactivity at that point by the reciprocal of the calculated photon attenuation at that point, one can estimate the amount of signal one would have received from that location if there were no photon attenuation. This correction can, in turn, be applied for every location within the object. If this correction is applied to the object with a uniform
radioactivity distribution, a uniform signal throughout the object would be obtained. This method, referred to as the first-order Chang correction for photon attenuation, is the most common approach to applying uniform attenuation correction in clinical SPECT (5).
This uniform assumption, however, is not at all appropriate for the thorax where there is lung tissue, spine, and mediastinum in addition to soft tissue, all of which have very different attenuating properties. If a uniform attenuation assumption was used, one would overcorrect the regions of the lungs and undercorrect the regions near the spine and mediastinum. This is of particular concern for myocardial SPECT. For these reasons, no attenuation correction was applied traditionally for cardiac SPECT since no correction was considered better than a poor correction. However, over the past 10 years, a number of investigators have implemented various approaches to non-uniform attenuation correction. In these cases, one needs to not only know the body outline, but also must know the types of tissues within that outline and their attenuation characteristics. To determine this, one acquires a transmission image using an external, photon-emitting source. These data indicate which regions within the body are highly attenuating and which yield less attenuating. This knowledge is incorporated into the SPECT reconstruction process to correct for the nonuniform attenuation. A variety of ways have been developed for the acquisition of the transmission data, several of which will be discussed in the section on instrumentation. In addition to attenuation correction, several other corrections have been developed for SPECT in order to provide more quantitative reconstructed data including those for scatter and resolution recovery.
SPECT INSTRUMENTATION
SPECT requires the ability to acquire projection images from a number of viewing angles about the patient. Thus the gamma camera must be mounted onto a gantry that allows the camera to rotate about the patient in a circular or elliptical orbit. One of the limitations of the rotating gamma camera is the inherently low sensitivity of the system. Since the cameras must utilize absorptive collimation to determine the directionality of the interacting

COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION |
281 |
Figure 4. Dual-detector SPECT camera. With this camera, the two detectors can be oriented at 1808 to each other for whole body imaging (a) or 908 for cardiac imaging (b).
photons, only a very small fraction of the photons emitted from within the patient will actually be detected by the gamma camera. More stringent collimation can be used to improve the spatial resolution but at a cost of lower sensitivity leading to a higher level of noise in the images. One straightforward approach to improving the sensitivity is to increase the number of detectors. Thus, both dual-detector and triple-detector SPECT systems have been developed. Figure 4a shows a modern dual-detector SPECT system.
Since the heart is located in the left anterior portion of the chest and low energy radiopharmaceuticals, such as thallium-201, are routinely used in this application, it is common to acquire cardiac SPECT data only over 1808 (from right anterior oblique to left posterior oblique) rather than over 3608, since most of the data that is acquired in the right posterior projection only adds noise and poor resolution to the image. This being the case, the use of two opposing detectors does not reduce the total imaging time, since one will still need to rotate the gantry over 1808. For this reason, a number of manufacturers have designed their dual-detector cameras such that the data can be acquired with the detectors either opposing each other (1808 orientation) and in a 908 orientation as shown in Fig. 4b. This allows for the same amount of SPECT data to be acquired in half the time. Increasing the number to three detectors improves the sensitivity of the SPECT device even further. Such triple-detector devices are excellent for acquiring SPECT but lack the flexibility for other nuclear imaging and thus tend to be less popular than the dual-detector systems.
As discussed previously, a transmission scan must be acquired in order to perform non-uniform attenuation correction. Several different approaches have been developed for the acquisition of the transmission image. Collimated, radioactive line sources can be scanned over an area of the patient during the acquisition of the emission data. Since the radionuclide in the sources (153Gd) emits a
gamma ray with a slightly different photon energy than the radiopharmaceutical administered to the patient, the two data sets (emission and transmission) can be acquired simultaneously. In an alternate method, a series of smaller line sources are used. These also contain 153Gd and thus again the emission and transmission data can be acquired simultaneously.
In the past several years, hybrid SPECT–CT systems have been developed. In these devices, a helical CT study is acquired in conjunction with the SPECT study on the same device. The CT scan is used to characterize the material within the body such that a non-uniform attenuation correction can be applied. It typically requires a transformation to be performed between the CT values and the attenuating coefficients for SPECT because the energies of the photons in CT are different that those for SPECT. In some cases, the device provides a diagnostic quality CT that can be interpreted either in conjunction with the SPECT study or independently. In the case of one SPECT–CT device, the CT provided is not of diagnostic quality, and it is used only for attenuation correction and gross anatomical correlation.
With the newest developments in molecular medicine has come the desire to image small animals such as rodents. Thus a number of investigators have developed methods of performing SPECT imaging in rodents with very high spatial resolution. One approach that is straightforward is the use of very small pinhole collimators. The size of pinhole is only 1 mm as compared to the 4–6-mm pinholes that are typically used for clinical imaging. This method can provide SPECT images that have spatial resolution that is better than what is typical in clinical SPECT by almost a factor of 10. These high resolution approaches cannot be applied in clinical SPECT because these very small pinholes are very inefficient and thus an inordinate image time on the order of several hours would be required in order to obtain human images of sufficient image quality.
282 COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION
CLINCIAL APPLICATIONS IN SPECT
In this section, we will review several of the most common clinical applications of SPECT.
Cardiac
The most common clinical indication for SPECT is myocardial (cardiac) perfusion imaging. Atherosclerotic heart disease is manifested by coronary artery narrowing, which limits blood flow to the region of the heart supplied by that artery, producing chest pain or myocardial infarction. SPECT can confirm or exclude significant coronary disease. If abnormal, invasive coronary angiography may be performed in anticipation of intervention, for example, coronary angioplasty or bypass surgery.
The radiopharmaceutical, thallium-201 or newer tech- netium-labeled cardiac radiotracers, is delivered by the individual coronary arteries to the myocardium where it is extracted. The SPECT images depict the 3D blood flow to each region of the myocardium supplied by its coronary artery.
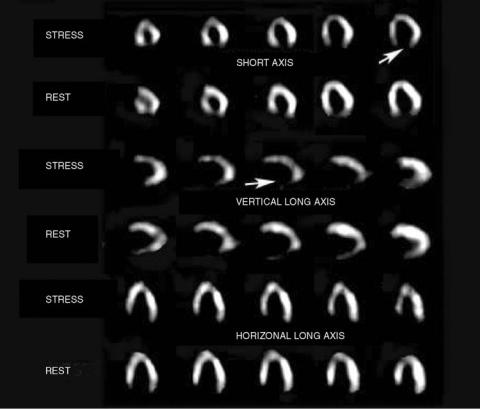
The study is performed in two stages, at rest and with stress. Treadmill exercise is the usual stress, although pharmacologic methods are used in those unable to exercise. In a normal heart, SPECT will show good blood flow at rest and stress. In a patient with a prior myocardial infarction, no blood flow will be seen in the nonviable region at either stage. A patient with significant coronary artery stenosis, without infarction, will have a normal rest study, but an abnormal exercise study (Fig. 5).
Adequate blood flow and oxygen can be delivered to a resting heart even with a high grade coronary artery obstruction, however, the increased demand for oxygen and blood flow required at stress cannot be met and myocardial uptake will be decreased in the myocardium fed by that artery.
Tumors
SPECT with various radiopharmaceuticals provide valuable information regarding the extent of disease and distribution in the body. This information is used for initial diagnosis, staging, preoperative localization, and evaluating response to therapy.
Gallium-67 citrate (67Ga) has been used for several decades for tumor imaging. It binds nonspecifically to a variety of tumors. Gallium-67 SPECT is used most commonly for imaging malignant lymphoma, a disease of lymphatic tissue seen in adults and children. Images depict the extent of disease and the effectiveness of therapy. This SPECT imaging is more accurate than CT or magnetic resonance imaging (MRI) for determining the effectiveness of therapy.
111In OctreoScan is a somatostatin receptor peptide imaging agent, most useful for imaging tumors of neuroendocrine origin, (carcinoid, gastrinoma, neuroblastoma, pheochromocytoma, etc.) SPECT cross-sectional imaging makes it possible to see small tumors that are often not detected with CT or MRI.
111In ProstaScint is radiolabeled monoclonal antibody directed against antigens on the surface of prostate cancer cells. It can detect the site of recurrence of prostate cancer,
Figure 5. The SPECT exercise and rest cardiac study. Short axis, vertical long axis, and horizontal long axis cross-sectional sequential slices are shown. Arrows point to the inferior wallwhere thereisnoperfusion.Since this finding is unchanged between stress and rest, this is diagnostic of an inferior wallmyocardial infarction.
suggested by a rising prostate serum antigen (PSA) level. Adequate imaging is not possible without SPECT because of the relatively high background activity.
Brain Imaging
SPECT is mandatory for brain imaging in order to visualize the various overlying and underlying convoluted regions of the brain.
Seizures are often caused by small regions of scar tissue in the brain. Patients not responding to drug therapy for their seizure disorder may be helped with resection of the seizure focus. Proper localization of the seizure site is critical for effective surgery. Brain wave studies (electroencephalogram or EEG) are only moderately successful in locating a seizure site. Even then, additional studies are needed for confirmation. The traditional method is to place electrodes on the surface of the brain and record electrical activity. However, this requires a neurosurgical operation and is associated with some morbidity.
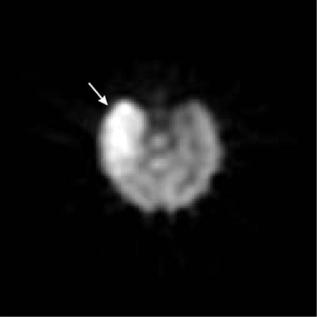
SPECT brain blood flow radiopharmaceuticals, 99mTc hexamethyl propylene amine oxime (99mTc HMPAO) and 99mTc ethyl cysteinate dimer (99mTc ECD), show the distribution of blood flow in the brain. During seizure activity, the small area of the brain responsible has increased metabolism and increased blood flow. Between seizures, the abnormal site has decreased metabolism and blood flow. SPECT imaging can localize the seizure site by detecting these focal abnormal blood flow patterns. An example of a SPECT study in a seizure patient is shown in Fig. 6.
The second indication is to determine the cause for dementia. Characteristic patterns of abnormal perfusion are seen with certain types of dementia, for example, Alzheimer’s disease, frontal lobe dementias, and multiinfarction dementia (strokes).
Figure 6. Brain SPECT study acquired during an epileptic seizure. The arrow indicates a region of high blood flow that corresponds to the part of the brain where the seizure originated.
COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION |
283 |
Bone
SPECT is used for a variety of indications. It can help detect small sites of tumor, fracture, or infection not easily seen or localized with traditional two-dimensional bone scanning. Precise localization of a bone radiopharmaceutical uptake (e.g., 99mTc diphosphonate), can help differentiate benign from malignant processes, confirm small fractures as the cause of pain, and detect sites of infection.
SUMMARY
SPECT is a 3D imaging approach for evaluating the physiology and function of the patient. The patient is injected with a small amount of a radiopharmaceutical and a series of projection images are acquired at different angles about the patient. These data are reconstructed into a series of cross-section views of the in vivo distribution of the radiopharmaceutical. Depending on the radiopharmaceutical used, the nuclear medicine physician can infer essential information regarding the patient’s physiologic condition. The rotating gamma camera is the most common device used to acquire SPECT data. Recent developments include the incorporation of CT into a hybrid SPECT–CT imaging device and the use of very small pinhole collimators for the imaging of small animals. With the advancements of molecular approaches to medicine, SPECT will continue to be a very important approach to medical imaging.
BIBLIOGRAPHY
1.Kuhl DE, Edwards RQ. Image separation radioisotope scanning. Radiology 1963;80: 653–661.
2.Kuhl DE, Edwards RQ. The Mark 3 Scanner: a compact device for multiple-view and section scanning of the brain. Radiology 1970; 96:563–70.
3.Keyes JW, Jr., Orlandea N, Heetderks WJ, Leonard PF, Rogers WL. The Humongotron—a scintillation-camera transaxial tomograph. J Nucl Med 1977; 18:381–387.
4.Jaszczak RJ, Murphy PH, Huard D, Burdine JA. Radionuclide emission computed tomography of the head with 99mTc and a scintillation camera. J Nucl Med 1977; 18:373–380.
5.Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci 1978; NS-25: 638–643.
Further Reading
Tsui BM. The AAPM/RSNA physics tutorial for residents. Physics of SPECT. Radiographics 1996; 16:173–183.
Miller TR. The AAPM/RSNA physics tutorial for residents. Clinical aspects of emission tomography. Radiographics 1996; 16:661–668.
Tsui BM, Frey EC, LaCroix KJ, Lalush DS, McCartney WH, King MA, Gullberg GT. Quantitative myocardial perfusion SPECT. J Nucl Cardiol 1998; 5:507–522.
Madsen MT. The AAPM/RSNA physics tutorial for residents. Introduction to emission CT. Radiographics 1995; 15:975–991.
King MA, Tsui BM, Pan TS. Attenuation compensation for cardiac single-photon emission computed tomographic imaging: Part 1. Impact of attenuation and methods of estimating attenuation maps. J Nucl Cardiol 1995; 2:513–524.
