
- •VOLUME 2
- •CONTRIBUTOR LIST
- •PREFACE
- •LIST OF ARTICLES
- •ABBREVIATIONS AND ACRONYMS
- •CONVERSION FACTORS AND UNIT SYMBOLS
- •CARBON.
- •CARDIAC CATHETERIZATION.
- •CARDIAC LIFE SUPPORT.
- •CARDIAC OUTPUT, FICK TECHNIQUE FOR
- •CARDIAC OUTPUT, INDICATOR DILUTION MEASUREMENT OF
- •CARDIAC PACEMAKER.
- •CARDIAC OUTPUT, THERMODILUTION MEASUREMENT OF
- •CARDIOPULMONARY BYPASS.
- •CARDIOPULMONARY RESUSCITATION
- •CARTILAGE AND MENISCUS, PROPERTIES OF
- •CATARACT EXTRACTION.
- •CELL COUNTER, BLOOD
- •CELLULAR IMAGING
- •CEREBROSPINAL FLUID.
- •CHEMICAL ANALYZERS.
- •CHEMICAL SHIFT IMAGING.
- •CHROMATOGRAPHY
- •CO2 ELECTRODES
- •COBALT-60 UNITS FOR RADIOTHERAPY
- •COCHLEAR PROSTHESES
- •CODES AND REGULATIONS: MEDICAL DEVICES
- •CODES AND REGULATIONS: RADIATION
- •COGNITIVE REHABILITATION.
- •COLORIMETRY
- •COMPUTERS IN CARDIOGRAPHY.
- •COLPOSCOPY
- •COMMUNICATION AIDS FOR THE BLIND.
- •COMMUNICATION DEVICES
- •COMMUNICATION DISORDERS, COMPUTER APPLICATIONS FOR
- •COMPOSITES, RESIN-BASED.
- •COMPUTED RADIOGRAPHY.
- •COMPUTED TOMOGRAPHY
- •COMPUTED TOMOGRAPHY SCREENING
- •COMPUTED TOMOGRAPHY SIMULATOR
- •COMPUTED TOMOGRAPHY, SINGLE PHOTON EMISSION
- •COMPUTER-ASSISTED DETECTION AND DIAGNOSIS
- •COMPUTERS IN CARDIOGRAPHY.
- •COMPUTERS IN THE BIOMEDICAL LABORATORY
- •COMPUTERS IN MEDICAL EDUCATION.
- •COMPUTERS IN MEDICAL RECORDS.
- •COMPUTERS IN NUCLEAR MEDICINE.
- •CONFOCAL MICROSCOPY.
- •CONFORMAL RADIOTHERAPY.
- •CONTACT LENSES
- •CONTINUOUS POSITIVE AIRWAY PRESSURE
- •CONTRACEPTIVE DEVICES
- •CORONARY ANGIOPLASTY AND GUIDEWIRE DIAGNOSTICS
- •CRYOSURGERY
- •CRYOTHERAPY.
- •CT SCAN.
- •CUTANEOUS BLOOD FLOW, DOPPLER MEASUREMENT OF
- •CYSTIC FIBROSIS SWEAT TEST
- •CYTOLOGY, AUTOMATED
- •DECAY, RADIOACTIVE.
- •DECOMPRESSION SICKNESS, TREATMENT.
- •DEFIBRILLATORS
- •DENTISTRY, BIOMATERIALS FOR.
- •DIATHERMY, SURGICAL.
- •DIFFERENTIAL COUNTS, AUTOMATED
- •DIFFERENTIAL TRANSFORMERS.
- •DIGITAL ANGIOGRAPHY
- •DIVING PHYSIOLOGY.
- •DNA SEQUENCING
- •DOPPLER ECHOCARDIOGRAPHY.
- •DOPPLER ULTRASOUND.
- •DOPPLER VELOCIMETRY.
- •DOSIMETRY, RADIOPHARMACEUTICAL.
- •DRUG DELIVERY SYSTEMS
- •DRUG INFUSION SYSTEMS
10.Liu J, Lu Y. Accelerated color change of gold nanoparticles assembled by DNAzymes for simple and fast colorimetric Pb2þ
detection. J Am Chem Soc 2004;126:12298–12305.
11.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997;277:1078–1081.
12.Long ME, Swofford RL, Abrecht AC. Thermal lens technique: A new method of absorption spectroscopy. Science 1976;191:
13.Sittampalam GS, Kahl SD, Janzen WP. High-throughput screening: advances in assay technologies. Cur Opin Chem Biol 1997;1:384.
14.Ihara T, Tanaka S, Chikaura Y, Jyo A. Preparation of DNAmodified nanoparticles and preliminary study for colorimetric SNP analysis using their selective aggregations. Nucleic Acids Res 2004;32:e105.
15.Liang RQ, Tan CY, Ruan KC. Colorimetric detection of protein microarrays based on nanogold probe coupled with silver enhancement. J Immunol Methods 2004;285:157–163.
16.Van Kampen EJ, Zijlstra WG. Spectrophotometry of hemoglobin and hemoglobin derivatives. Adv Clin Chem 1983;23:199.
17.Henry RJ, Golub OJ, Berkman S, Segalove M. Critique on the Icterus index determination. Am J Clin Pathol 1953;23:841.
18.Kingsley GR. Procedure for serum protein determinations. Stand Methods Clin Chem 1972;7:199.
19.Lowry OH, Rosebrough NS, Farr AL, Randall RI. Protein measurement with the Folin phenol reagent. J Biol Chem 1951;193:265.
20.Rylatt DB, Parish CR. Protein determination on an automatic spectrophotometer. Anal Biochem 1982;121:213.
21.Peats S. Quantitation of protein and DNA in silver stained gels. Anal Biochem 1984;140:178.
22.Jin L-T, Choi J-K. Usefulness of visible dyes for the staining of protein or DNA in electrophoresis. Electrophoresis 2004;25: 2429.
23.Finlay GJ, Baguley BC, Wilson WR. A semiautomated microculture method for investigating growth inhibiting effects of cytotoxic compounds on exponentially growing carcinoma cells. Anal Biochem 1984;139:272.
24.Webster D. The immediate reaction between bromcresol green and serum as a measure of albumin content. Clin Chem (Winston-Salem, NC) 1977;23:663.
25.Stookey LL. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem 1970;42:779.
26.Jendrassik L, Grof P. Simplified photometric methods for the determination of the blood bilirubin. Biochem Z 1938; 297:81.
27.Lowry O, Passonneau J. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. Chapt. 5.
28.Fawaz EN, Roth L, Fawaz G. The enzymatic estimation of inorganic phosphate. Biochem Z 1966;344:212.
29.Morris DL, Ellis PB, Carrico HJ, Yeager RM, Schroeder HR, Schroeder JP, Albarella JP, Boguslaski RC. Flavin adenine dinucleotide as a label in homogeneous colorimetric iminunoassays. Anal Chem 1981;53:658.
30.Fossati P, Prencipe L. Serum triglycerides determined colorimotrically with an enzyme that produces hydrogen peroxide. Clin Chem (Winston-Salem, NC) 1982;28:2077.
31.Ewen LM, Spitzer RW. Improved determination of prostatic acid phosphatase (sodium thymolphthalein monophosphate substrate). Clin Chem (Winston-Salem, NC) 1976;22:627.
32.Babson AL, Philips GE. A rapid colorimetric assay for serum lactic dehydrogenase. Clin Chim Acta 1965;12:210.
33.Szasz G, Gruber W, Bernt E. Creatine kinase in serum.
1.Determination of optimum reaction conditions. Clin Chem (Winston-Salem, NC) 1976;22:650.
COLPOSCOPY 197
34.Hoever M, Zbinden P. The evolution of microarrayed compound screening. Drug Discover Today 2004;9:358.
35.Hargis LG, Howell JA, Sutton RE. Ultraviolet and light absorption spectrometry. Anal Chem 1996;68:169R–183R.
36.Rooze H. Kinetic-spectrophotometric determination of trace levels of iron. Anal Chem 1984;56:601.
Further Reading
Evenson MA. Chapt. 3: Spectrophotomtric Techniques. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of Clinical Chemistry. 3rd ed. Philadelphia (PA): W.B. Saunders; 1999. p 75–93. A widely used reference and textbook for clinical chemists. Chapter 3 covers both the concepts and the instrumentation of spectrophotometry.
Hargis LG, Howell JA, Sutton RE. Ultraviolet and light absorption spectrometry. Anal Chem 1996;68:169R–183R. A review includes a comprehensive list of reagents used for the spectrophotometric analysis of organic and inorganic compounds. It also includes a brief description of data processing and instrumentation. A total of 440 literatures are cited in this article.
Mikkelsen SR, Corton E. Bioanalytical Chemistry. Hoboken (NJ): John Wiley & Sons Inc., 2004. Chapt. 1. Spectroscopic Methods for Matrix Characterization. Chapt. 2. Enzyme. These two chapters focus on the biochemical and biomedical applications, especially to the spectroscopic measurement of protein concentrations and enzyme kinetics.
Pesce AJ, Frings CS, Gauldie J. Chapt. 4. Spectral Techniques. In: Kaplan LA, Pesce AJ, Kazmierczak SC, editors. Clinical Chemistry: Theory, Analysis, Correlation. 4th ed. St. Louis (MO): Mosby; 2003. p 83–106. This chapter includes a good description on the design of various types of spectrophotometers.
Skoog DA, West DM, Holler FJ, Crouch SR. Fundamentals of Analytical Chemistry. Brooks/Cole: 2004. A textbook in analytical chemistry with extensive coverage of both the principles and applications. Part V, Spectrochemical Analysis, includes: Chapt. 24. Introduction to Spectrochemical Analysis; Chapt. 25. Instruments for Optical Spectroscopy; and Chapt. 26: Molecular Absorption Spectroscopy.
See also ANALYTICAL METHODS, AUTOMATED; FLUORESCENCE MEASUREMENTS.
COMPUTERS IN CARDIOGRAPHY. See ELECTRO-
CARDIOGRAPHY, COMPUTERS IN.
COLPOSCOPY
MOSTAFA A. SELIM
ABDELWAHAB D. SHALODI
Cleveland Metropolitan
General Hospital
Palm Coast, Florida
Today, fewer women die annually from carcinoma of the cervix then in any period in modern history. Increased utilization of cervical cytology (Pap smears) and better assessment and evaluation of patients with abnormal Papsmearsareimportantreasonsfortheprogressinthisfield.
The Papanicolaou stained cytology smear is an invaluable tool for detecting cervical carcinoma. Such a test
198 COLPOSCOPY
depends on collection of exfoliating cells from the cervix, spreading the material on a glass slide, and staining with special stains in order to identify the microscopic structures in nuclei and the cytoplasm of the cell. An abnormal smear is called positive and a normal smear is called negative for malignancy. However, in recent years the incidence of false-negative smears has been recognized to be significant, varying between 15 and 22% (1–4). This high incidence of false-negative smears emphasizes the need for histological study of the abnormal cervix before any therapy is started, regardless of the findings from the cytology smear.
In order to fill the serious gap in the screening of cervical cancer and its precursors created by the high incidence of false-negative smears, colposcopy is utilized (3–6).
THE COLPOSCOPE
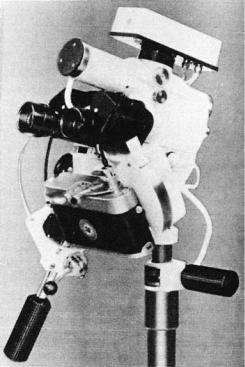
The word colposcopy simply means viewing the vagina. The colposcope is a binocular, long-focal-length, wide-field microscope with which it is possible to examine the epithelium and subepithelial vascular pattern of the cervix at magnifications varying from 6 to 40 . Figure 1 shows a typical instrument. A beam of light is projected between the objectives so that the cervix is well illuminated. To help the visualization of blood vessels, a green filter may be fitted to the light source. This adds contrast in viewing microvessels against the tissue. Photographic apparatus consisting of a prefocused lens system, a 35 mm camera, and a flash tube are attached to the colposcope in order to document the findings. The photographs obtained using
Figure 1. Colposcope with camera and flash attached.
35 mm film are two dimensional (2D). To obtain threedimensional (3D) images, the instrument may be fitted with a stereocamera that produces image pairs. These pairs give a stereoscopic effect when viewed in a special viewer. The colposcope, the camera, and the flash tube are mounted on a fixed, sturdy base with rack-and-pinion drives for fine positioning and focusing. Modern coloposcope is equipped with digital video camera interfaced microcomputer.
FINDINGS
A colposcopic examination is considered satisfactory whenever it is possible to view all of the critical portions of cervical anatomy, and if an abnormal lesion is seen, the upper margin of this lesion must be adequately visualized. Specifically, it is necessary to view the entire transformation zone. The transformation zone is the lower segment of the endocervical canal. This area is normally covered by columnar epithelium, although through the process of metaplasia some areas or the entire zone can be transformed into squamous epithelium. The normal transformation zone is recognized by the presence of small glandular openings, nabothian cysts, and a normal pattern.
The procedure can be performed quickly and easily on out-patients without anesthesia. However, the gynecologist needs intensive training in order to master the interpretation of what they sees.
The colposcope was first devised by Hinselmann in 1925 at Hamburg, Germany. The instrument became popular in the German speaking and Latin nations. It was accepted in the English speaking nations in the 1960s. One of the reasons for the delay in accepting colposcopy was that all of the original studies and the terminology were written in German (7,8).
INDICATIONS FOR COLPOSCOPY (3–6)
Ideally all patients, during their gynecological examination, at one time or another ought to be examined colposcopically. However, since colposcopy is time consuming and requires specialized training, this is usually not possible, and thus the following indications are recommended:
1.All patients with abnormal cervical cytology.
2.All patients with an abnormal lesion on the cervix, vulva, or vagina.
3.Cases of persistent cervicitis, vulvitis, or vaginitis.
4.Pregnant patients with unexplained vaginal bleeding.
5.All offspring of diethylstilbestrol-exposed pregnancies.
Colposcopy plays an essential role in evaluating these patients. The colposcope helps to pinpoint the abnormal area. However, the final diagnosis has to await histopathological diagnosis. This tissue diagnosis is obtained through endocervical curettings, punch biopsy, and/or cone biopsy according to the individual case, as illustrated in Fig. 2.
Recent studies confirmed that colposcopy could be utilized in follow-up in conservative management of low grade

COLPOSCOPY 199
precancerous lesions, as most of them regress on its own. In addition, the colposcope can help in detecting and guiding treatment of genital infections.
A movable, counterweighted arm is used for rough positioning of the colposcope. The arm is fixed to the examining table or to a heavy wheeled base (5,6).
There are several commercially available colposcopes. Manipulation, magnification, length, intensity, and type of green filter vary from one instrument to another. For detailed inspection of the vascular pattern, the magnification ought to be not <14 and preferably 16 (7,8).
TECHNIQUE OF COLPOSCOPY
1.The cervix and the upper vagina are examined, at a magnification of not < 14 , after excess mucus is removed by cotton swab moistened by physiological saline, which allows the subepithelial architecture to be seen in greater detail. To enhance visibility the green filter should be used.
2.Acetic acid (3%) is gently applied by a cotton swab. Abnormal epithelium will become whitish and sharply demarcated. The normal squamous epithelium appears pink, and columnar epithelium will have a grape-like appearance. The effect of the acetic acid will last 30–40 s.
3.Endocervical curetting and punch biopsies should be done for all unusual-appearing areas. Specimens should be put in separate formalin-containing bottles.
4.Bleeding is usually minimal and does not need any packing. However, during pregnancy, when vascularity is increased, Oxycel, Surgicel packing, or the use of Munsell’s solution may be necessary.
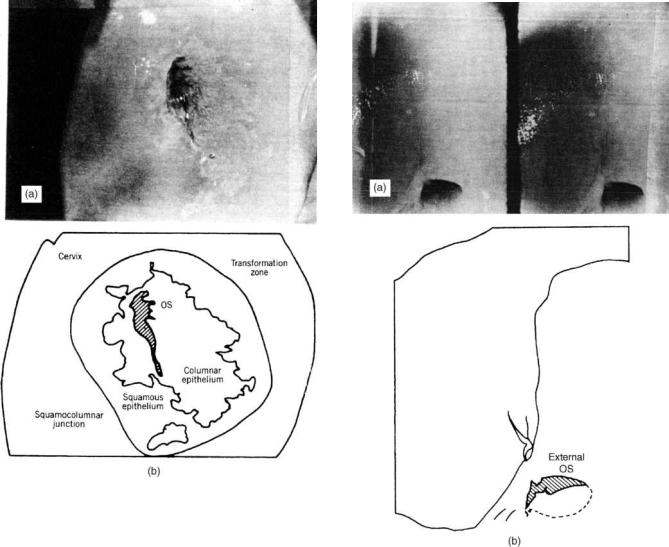
Normal pattern of blood vessels. An example of a satisfactory colposcopic finding is shown in Fig. 3. The sketch identifies the various features of this view of the cervix. An example of an unsatisfactory view of the cervix is shown
Figure 2. Protocol for management of patients.
in Fig. 4. In this case the transformation zone as indicated in the sketch is not fully visualized; the columnar epithelium is not seen and/or the upper margin of the lesion extends into the endocervical canal and thus cannot be fully visualized.
In evaluating satisfactory colposcopic findings, many factors must be considered. It is beyond the scope of this article to provide a detailed description of the different patterns seen in colposcopy, but a general idea of what is looked for can be presented. In order to reach an adequate diagnosis, the colposcopist must consider the following criteria in observing the uterine cervix: (1) vascular pattern, (2) intercapillary space, (3) color and texture, (4) surface pattern, and (5) sharpness of the line of demarcation between the lesion and the rest of the cervix.
The lower segment of the endocervical canal that extends to the visible part of the cervix is the transformation zone. This area is originally and normally covered by columnar epithelium. Through a process of metaplasia, some areas or the whole zone can be transferred into squamous epithelium. The normal transformation zone is recognized by the presence of small glandular openings, nabothian cysts, and a normal pattern of blood vessels (Fig. 3).
Vascular Pattern
Normal epithelium vessels appear as fine dots or a network of capillaries. However, in abnormal pathology, the individual vessel becomes prominent, leading to punctuation (Fig. 5). A mosaic pattern is due to increased communication between the individual vessels, arranged parallel to the surface epithelium (Fig. 6). Atypical vessels are capillaries that are irregular in shape, size, course, and arrangement (Fig. 7).
Intercapillary Space. Due to rapid proliferation of the abnormal epithelium, the intercapillary distance between the vessels is increased. The intercapillary distance of normal capillaries varies between 50 and 250 mm with an average of 100 mm. The intercapillary distance in early
200 COLPOSCOPY
Figure 3. (a) Areas of glandular epithelium extending to ectocervix became whitish upon addition of acetic acid. Note the grape-like appearance of the glandular epithelium; no abnormal vessels or lesions are seen, and the lower end of the cervical canal is adequately visualized. (b) Sketch of the transformation zone, consisting of columnar and squamous epithelium. The squamous– columnar junction is part of the transformation zone. In the lower left corner of the diagram there is an island of glandular epithelium in the middle of the squamous epithelium. Note that the color of the squamous epithelium in the transformation zone is identical to that of the original squamous epithelium.
intraepithelial neoplasia (CIN 1) is 200 mm, while in severe intraepithelial neoplasia (CIN 3) it is 450–550 mm (6) (Figs. 5 and 6).
Color Tone. Abnormal epithelium is darker than normal epithelium, and upon addition of acetic acid it temporarily becomes whitish (Figs. 5 and 6).
Surface Epithelium. Abnormal lesions are uneven, granular, papillomatous, or nodular (Figs. 5–7). Normal squamous epithelium is smooth, whereas columnar epithelium has a grape-like appearance (Fig. 3).
Line of Demarcation. The abnormal epithelium is usually raised and well demarcated from the normal epithelium, especially after addition of acetic acid (Figs. 3–7).
Figure 4. (a) Unsatisfactory view of the cervix and (b) accompanying sketch. This cervix is covered completely by squamous epithelium; no glandular epithelium is seen, including the lower endocervical canal. The left side of the cervix has a large red region. This region is covered by newer squamous epithelium, which is more transparent so that the normally arranged vessels are seen though it. The entire field of the figure is covered by squamous epithelium. Neither glandular epithelium nor the squamocolumnar junction can be seen. This represents an unsatisfactory colposcopic examination.
ACCESSORY INSTRUMENTS TO COLPOSCOPY
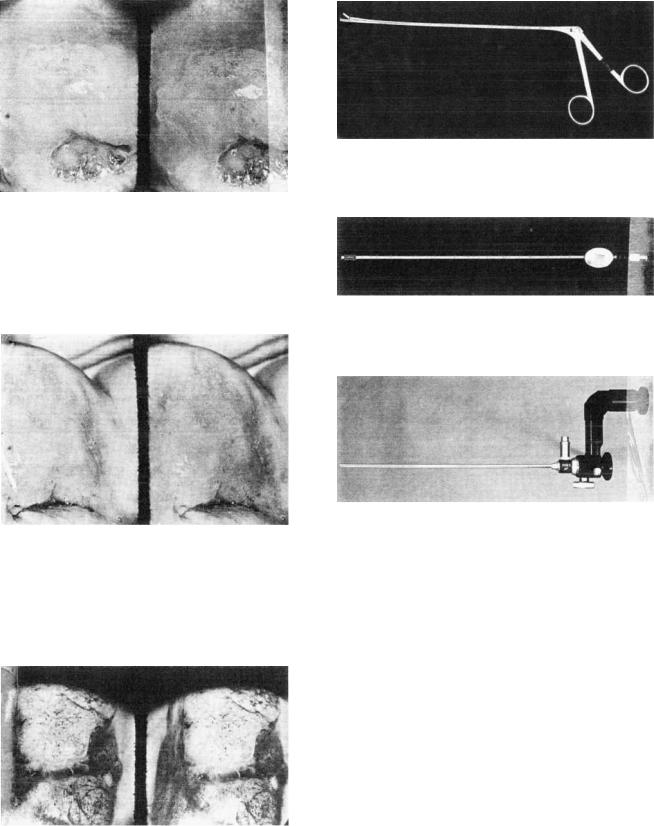
The best examination table is one that can be adjusted for height and tilt, is in the lithotomy position, and is preferably electrically operated. Punch biopsy forceps ought to be sharp with square jaws (Fig. 8) in order to obtain welloriented, adequate tissue for pathological examination. In addition, in order to prevent loss of fragments of tissue obtained by endocervical curettings, the curette must be a closed-sided instrument to create negative suction (Fig. 9).
To aid in visualization of the endocervical canal, which cannot be seen by a colposcope, a Hamou microcolpohysteroscope can be utilized (Fig. 10). The technique utilizes
COLPOSCOPY 201
Figure 5. Punctation. Large, well-defined areas of the transformation zone became white after addition of acetic acid. The areas are covered by punctate of blood vessels with wide intercapillary distances. These abnormal lesions do not extend into the canal, and the lower canal columnar epithelium is seen and is normal. Some glandular openings are seen in the lower right part of the figure.
Figure 6. Large transformation zone covered by a mosaic pattern, inside well-defined borders. The areas became whitish after addition of acetic acid. The upper margin of the abnormal lesion can be adequately seen. The columnar epithelium is seen and appears to be normal. These findings are typical for carcinoma in situ, a precancerous lesion.
Figure 7. Highly abnormal cervix covered by atypical vessels with increased intercapillary distance. It is nodular and shows an exophytic growth pattern with a whitish and glossy appearance. These findings are indicative of invasive carcinoma.
Figure 8. Punch biopsy forceps. Note the square jaw necessary to obtain an adequate and well-oriented biopsy for pathological examination.
Figure 9. Curette. Note that it is serrated and open only from one side in order to prevent loss of tissue. The stem is hollow to create a suction effect.
Figure 10. Hamou microcolpohysteroscope. Note that it has the same caliber as the curette and has an outlet for a fiberoptic light source.
the contact technique, thereby achieving 60 magnification, and does not need any transmission media other than the normal mucus secretion of the endocervix. This method can aid in assessing the extent of the disease and may aid in defining the extent of cone biopsy, whenever it is needed to investigate or to treat the disease (9).
Digital Colposcopy (10–12)
Digital colposcopy is an improvement on regular colposcopy. Integrating video camera interfaced microcomputer and using real time image achieve this. Such arrangement allows computerized manipulation of the image signal. In order to manipulate the image by the computer it needs to be converted into matrix number. Each number is representing one point in image matrixes, called pixel. Each pixel has a value that corresponds to discrete gray level. The computer then converts the number back to analogue for display on the video.
Regular colposcope uses green filter to enhance visualization of blood vessels and discoloration. However, this has its limitation. Digital colposcopy has different ways to
