
Molecular and Cellular Signaling - Martin Beckerman
.pdf
380 15. Apoptosis
The majority of chemotherapeutic approaches to killing tumors is based on the idea of selectively inducing apoptosis in the diseased cells. Many anticancer drugs target the mitochondria with the aim of producing a decrease in membrane potential leading to the efflux of pro-apoptotic molecules and the activation of the apoptosome/Caspase9 pathway. There are a number of ways that a decrease in mitochondrial membrane potential may be induced. One way is to supply ligands for either the ANT or the VDAC. Alternatively, drugs may be used that trigger increases in ROS leading to an increase in membrane permeability.
All of the major components of the apoptosis machinery are tightly controlled. Negative feedback loops ensure that small perturbations and random releases of Cytochrome c or Smac/DIABLO from the mitochondria do not set off apoptosis. Restorative processes in the form of antioxidant scavenger systems prevent ROS generated within the mitochondria from causing excessive damage. Calcium homeostasis within the cytosol and organelles such as the ER and mitochondria are under negative feedback control. Finally, the inner mitochondrial transmembrane potential Ym is a quantity with an important role in apoptosis and endowed with restorative properties. In order to be effective, therapeutic approaches that produce stresses and perturb the mitochondria must overcome the plethora of protective measures used by the cell to deal with such stresses. These issues compound the difficulties in dealing with machinery that doesn’t work quite right because of mutations in genes that encode its key elements.
References and Further Reading
General References
Igney FH, and Krammer PH [2002]. Death and anti-death: Tumor resistance to apoptosis. Nature Rev. Cancer, 2: 277–288.
Johnstone RW, Ruefli AA, and Lowe SW [2002]. Apoptosis: A link between cancer genetics and chemotherapy. Cell, 108: 153–164.
Mattson MP [2000]. Apoptosis in neurodegenerative disorders. Nature Rev. Mol. Cell Biol., 1: 120–129.
Meier P, Finch A, and Evan G [2000]. Apoptosis in development. Nature, 407: 796–801.
Vila M, and Przedborski S [2003]. Targeting programmed cell death in neurodegenerative diseases. Nature Rev. Neurosci., 4: 1–11.
Caspases
Boatright KM, et al. [2003]. A unified model for apical caspase activation. Mol. Cell, 11: 529–541.
Chai JJ, et al. [2001]. Crystal structure of a procaspase-7 zymogen: Mechanisms of activation and substrate binding. Cell, 107: 399–407.
Chang DW, et al. [2003]. Interdimer processing mechanism of procaspase-8 activation. EMBO J., 22: 4132–4142.

References and Further Reading |
381 |
Shi YG [2002]. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell, 9: 459–479.
Stennicke HR, and Salvesen GS [2000]. Caspases—Controlling intracellular signals by protease zymogen activation. Biochim. Biophys. Acta, 1477: 299–306.
Bcl-2 Proteins
Cheng EHYA, et al. [2001]. Bcl-2, BclxL sequester BH3 domain only molecules preventing BAXand BAK-mediated mitochondrial apoptosis. Mol. Cell, 8: 705–711.
Cory S, and Adams JM [2002]. The Bcl-2 family: Regulators of the cellular life-or- death switch. Nature Rev. Cancer, 2: 647–656.
Gross A, McDonnell JM, and Korsmeyer SJ [1999]. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev., 13: 1899–1911.
Letai A, et al. [2002]. Distinct BH3 only domain either sensitize or activate mitochondrial apoptosis, serving as prototypes cancer therapeutics. Cancer Cell, 2: 183–192.
Moreau C, et al. [2003]. Minimal BH3 peptides promote cell death by antagonizing anti-apoptotic proteins. J. Biol. Chem., 278: 19426–19435.
Scorrano L, et al. [2002]. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell, 2: 55–67.
DISC Signaling
Enari M, et al. [1998]. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature, 391: 43–50.
Nagata S [1997]. Apoptosis by death factor. Cell, 88: 355–365.
Scaffidi C, et al. [1998]. Two CD95(APO-1/Fas) signaling pathways. EMBO J., 17: 1675–1687.
Weber CH, and Vincenz C [2001]. The death domain superfamily: A tale of two interfaces? Trends Biochem. Sci., 26: 475–481.
Regulation of the Apoptotic Signaling Pathways by NF-kB
Karin M, and Lin A [2002]. NF-kB at the crossroads of life and death. Nature Immunology, 3: 221–227.
Mayo CY, et al. [1998]. NF-kB antiapoptosis: Induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase 8 activation. Science, 281: 1680–1683.
Permeability Transition Pore Complex
Crompton M [1999]. The mitochondrial permeability transition pore and its role in cell death. Biochem. J., 341, pt. 2: 233–249.
Ott M, et al. [2002]. Cytochrome c release from mitochondria proceeds by a twostep process. Proc. Natl. Acad. Sci. USA, 99: 1259–1263.
Susin SA, Zamzami N, and Kroemer G [1998]. Mitochondria as regulators of apoptosis: Doubt no more. Biochim. Biophys. Acta., 1366: 151–165.
Apoptosome Assembly
Acehan D, et al. [2002]. Three-dimensional structure of the apoptosome: Implications for assembly, pro-caspase-9 binding, and activation. Mol. Cell, 9: 423–432.

382 15. Apoptosis
Bratton SB, et al. [2001]. Recruitment, activation and retention of caspase-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J., 20: 998–1009.
Inhibitor of Apoptosis Proteins
Huang HK, et al. [2000]. The inhibitor of apoptosis, cIAP2, functions as a ubiquitinprotein ligase and promotes in vitro monoubiquitination of caspases 3 and 7. J. Biol. Chem., 275: 26661–26664.
Salvesen GS, and Duckett CS [2002]. IAP proteins: Blocking the road to death’s door. Nature Rev. Mol. Cell Biol., 3: 401–410.
Suzuki Y, Nakabayashi Y, and Takahashi R [2001]. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteosomal degradation and caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc. Natl. Acad. Sci. USA, 98: 8662–8667.
Yang Y, et al. [2000]. Ubiquitin protein ligase activity of IAPs and their degradation in proteosomes in response to apoptotic stimuli. Science, 288: 874–877.
IAP Counterregulators Smac/DIABLO and Omi/HtrA2
Holley CL, et al. [2002]. Reaper eliminates IAP proteins through stimulated IAP degradation and generalized translational inhibition. Nature Cell Biol., 4: 439–444.
Nicholson DW [2002]. Baiting death inhibitors. Nature, 410: 33–34.
Srinivasula SM, et al. [2001]. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase acytivity and apoptosis. Nature, 410: 112–116.
Yoo SJ, et al. [2002]. Hid, Ror and Grim negatively regulate DIAP1 levels through distinct mechanisms. Nature Cell Biol., 4: 416–424.
Mitochondrial Physiology
Duchen MR [2000]. Mitochondria and calcium: From cell signaling to cell death. J. Physiol., 529: 57–68.
Jacobson J, and Duchen MR [2002]. Mitochondrial oxidative stress and cell death in astrocytes—Requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci., 115: 1175–1188.
Kowaltowski AJ, Castilho RF, and Vercesi AE [2001]. Mitochondrial permeability transition and oxidative stress. FEBS Lett., 495: 12–15.
Marchetti P [1997]. Redox regulation of apoptosis: Impact of thiol oxidation status on mitochondrial function. Eur. J. Immunol., 27: 289–296.
Ricci JE, Gottlieb RA, and Green DR [2003]. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol., 160: 65–75.
ER and Calcium Signals to the Mitochondria
Boehning D, et al. [2003]. Cytochrome c binds to inositol (1,4,5) triphosphate receptors, amplifying calcium-dependent apoptosis. Nature Cell Biol., 5: 1051–1061.
Li C, et al. [2002]. Bcl-xL affects Ca2+ homeostasis by altering expression of inositol 1,4,5-triphosphate receptors. Proc. Natl. Acad. Sci. USA, 99: 9830–9835.
Nutt LK. et al. [2002]. Bax-mediated Ca2+ mobilization promotes cytochrome c release during apoptosis. J. Biol. Chem., 277: 20301–20308.

Problem 383
Orrenius S, Zhivotovsky B, and Nicotera P [2003]. Regulation of cell death: The calcium-apoptosis link. Nature Rev. Mol. Cell Biol., 4: 552–565.
Scorrano L, et al. [2003]. BAX and BAK regulation of endoplasmic reticulum Ca2+: A control point for apoptosis. Science, 300: 135–139.
p53 Regulation
Flores ER [2002]. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature, 416: 560–564.
Mihara M, et al. [2003]. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell, 11: 577–590.
Vousden KH, and Lu X [2002]. Live or let die: The cell’s response to p53. Nature Rev. Cancer, 2: 594–604.
Cancer Therapy
Herr I, and Debatin KM [2001]. Cellular stress response and apoptosis in cancer therapy. Blood, 98: 2603–2614.
Johnstone RW, Ruefli AA, and Lowe SW [2002]. Apoptosis: A link between cancer genetics and chemotherapy. Cell, 108: 153–164.
Kaufmann SH, and Earnshaw WC [2000]. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res., 256: 42–49.
Nicholson DW [2000]. From bench to clinic with apoptosis-based therapeutic agents. Nature, 407: 810–816.
Problem
15.1Some cell types are more susceptible to apoptosis than others. What classes of cells are especially responsive to apoptosis signals? What kinds of cells send out proapoptosis messages as part of their normal functions? Briefly describe some of the ways a cell has of regulating its own sensitivity to apoptosis.

16
Gene Regulation in Eukaryotes
The finding that metazoan genomes are as small as they are was remarked upon in Chapter 1. In the introductory chapter, several aspects of eukaryotic cell organization that make this possible were noted. One of the most important consequences of the changes in going from prokaryotic to eukaryotic cell organization was creation of many different ways of regulating gene and protein expression, and for fine-tuning and specializing the functions being performed. As a result of changes in the way the cells are organized and gene expression and protein function are regulated, large increases in complexity become possible without requiring large numbers of additional genes. This central aspect will be examined in detail in this chapter, which is devoted to the regulation of gene expression and protein synthesis. Transcription will be discussed first, followed by alternative splicing and translation.
Transcription in eukaryotes, even in the unicellular yeast, is more complex than in prokaryotes because of the presence of chromatin. Recall from Chapter 2 that eukaryotic DNA is highly condensed. Most of the chromatin is transcriptionally silent; that is, the DNA is tightly wound about the histone core and promoter sites are not accessible. The molecular machinery responsible for transcription not only contacts and manipulates the DNA molecule but also interacts with and alters the chromatin structure. The molecular machines involved in modifying the chromatin structure and transcribing genes are large. They can contain 50 or more subunits and have molecular masses of several megadaltons. An example of how large these machines may become is supplied by an examination of yeast cells. A set of complexes that might be found in a typical yeast cell is presented in Table 16.1.
The first kind of complex is the basal (core) transcription machinery, which includes RNA polymerase II and a set of general transcription factors. It binds at the transcription start site and catalyzes the polymerization of RNA chains from DNA templates. This complex is commonly referred to as the preinitiation complex (PIC). The second kind of protein complex is exemplified in yeast by a set of about 20 proteins collectively
385

386 16. Gene Regulation in Eukaryotes
TABLE 16.1. Protein complexes involved in transcription in yeast cells.
|
Number of |
Molecular mass |
|
Machine |
subunits |
(MDa) |
Function |
|
|
|
|
Basal transcription |
>40 |
2 |
Transcription |
|
|
|
driver (PIC) |
Mediator |
20 |
1 |
Signal integrator |
SWI/SNF complex |
11 |
2 |
Chromatin |
|
|
|
remodeler |
SAGA |
15 |
2 |
Histone modifier |
|
|
|
|
called the Mediator. Complexes similar to the Mediator have been found in all metazoans examined. They contain suppressor of RNA polymerase B (another name for RNA polymerase II) proteins (Srb proteins) and Mediator proteins (Med proteins), and serve as intermediaries between the transcription factors and the basal transcription machinery. The last two complexes modify chromatin by chemomechanical means. The first (SWI/SNF) breaks and reforms histone-DNA bonds, while the second (SAGA) alters the chemical affinity of histones for the DNA thereby either promoting or inhibiting transcription through acetylation of the histone tails. If all the contributions from the four complexes are combined the result is a machine with about 90 subunits and a molecular mass of 7 MDa. (A few subunits are shared by more than one complex but this changes the additive result only slightly.) These machines and the underlying mechanisms are highly conserved from yeast to man.
16.1 Organization of the Gene Regulatory Region
The molecular machines introduced in the last section carry out their functions at promoters. This is the name given to regions of DNA that contain binding sites for the transcription control elements and transcription start sites for RNA polymerase II (RNAP II). The core promoter is a region of approximately 40 bp that directs the start of transcription by the preinitiation complex. The core promoter contains several short DNA sequences that are recognized by DNA-binding proteins belonging to the PIC. One of these is the TATA box, an A/T rich, 8 bp sequence located 25 to 30 bp upstream of the start site for transcription. A second sequence known as initiator (Inr) is positioned at the start site. Other important sequences contained within the core promoter include the TFIIB recognition element
(BRE) and the downstream promoter element (DPE).
The core promoter correctly positions and orients RNAP II at the start site. The TBP subunit of the PIC recognizes the TATA box and the TFIIB subunit recognizes the BRE. The architecture of the core promoter in yeast and metazoans is depicted in Figure 16.1. Not all promoters contain TATA

16.2 How Promoters Regulate Genes |
387 |
FIGURE 16.1. Eukaryotic core promoter: Promoters are major endpoints of signaling pathways and have multiple sites for binding proteins. (a) The core promoter contains several binding sites for components of the basal transcription machinery to bind. The TATA box is located 25 to 30 bp upstream from the transcription start site, Inr, centered about the +1 position. The TFIIB recognition element (BRE) is located just above the TATA box while the downstream promoter element (DPE) is located about 30 bp from Inr. (b) The first event to occur is the binding of the TATA box binding protein (TBP) to the TATA box, followed by recruitment of TFIIB to the TBP and the BRE, followed by recruitment of RNA polymerase II and other elements of the PIC.
boxes. Both the TATA box and the Inr can direct transcription initiation by RNAP II. TATA box sequences are present in promoters for many abundantly transcribed genes. Housekeeping genes and genes encoding growth factors and transcription factors often have TATA-less promoters. They utilize the Inr for correct positioning of RNAP II at the start site. Some promoters, lacking both the TATA box and the Inr, rely on the DPE for positioning.
16.2 How Promoters Regulate Genes
One of the most important aspects of gene expression is the presence of multiple binding sites for regulatory proteins in the promoter. The core promoter was discussed in the last section. There are two other components— the proximal control region and the distal control region. These regions contain binding sites that are recognized by regulatory proteins in a sequence-specific manner. Several binding sites are usually found in the proximal region located just upstream of the core promoter. These binding sites are referred to as response elements. Proteins that stimulate transcription when they bind at these sites are called activators while those that impede transcription are called repressors. A third kind of protein, which
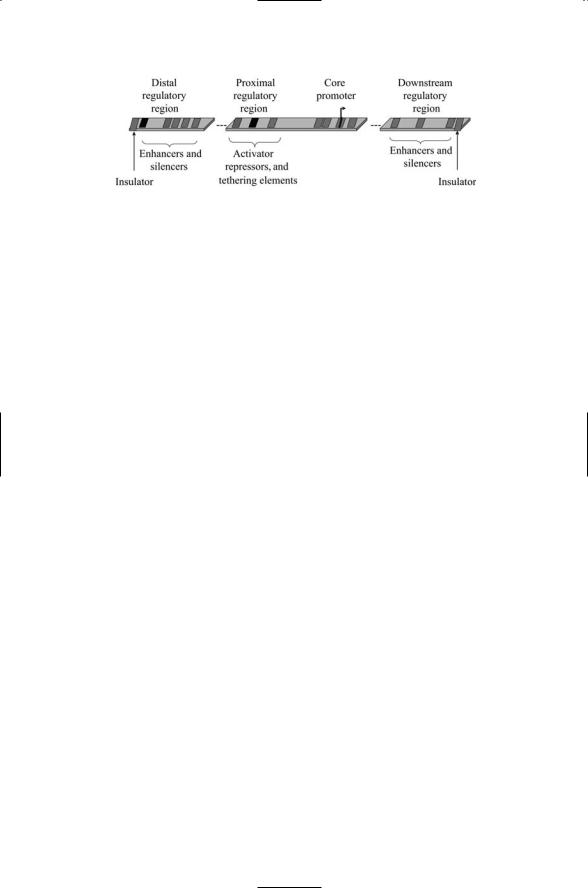
388 16. Gene Regulation in Eukaryotes
FIGURE 16.2. Eukaryotic promoter consisting of the core promoter plus proximal, distal, and downstream regulatory sequences called responsive elements (REs): The REs are represented in the figure as dark patches separated from the core promoter and from one another by spacers. A pair of insulators, one at each end, bounds the gene regulatory region.
provides sites for attachment of coactivators and corepressors and mediates long range enhancer-promoter interactions, is called a tethering element (Figure 16.2).
Distal sites can be located either upstream of the core promoter or downstream, in between coding regions and even inside introns. These are the sites where the long-range interactions between regulatory proteins and the basal transcription machinery take place. The positively acting proteins that bind the DNA are called enhancers and those that inhibit transcription are called silencers. The distal regulatory regions are generally larger than the proximal ones and supply binding sites for up to a dozen or so proteins.
Besides proximal and distal sites, a third kind of site—one that marks a boundary—is called an insulator. Insulators are DNA sequences that mark boundaries between independent sections of DNA. They protect the genes they delineate from influences outside the protected regions, serving as blockers of enhancer functions or as barriers against elements that inactivate chromatin. When situated between an enhancer and a promoter the insulators are able to block action of that enhancer on the promoter. They also block the actions of gene silencers that act on the chromatin, thereby marking the ends of chromatin domains (Figure 16.2).
Promoters are major endpoints of signaling pathways. Transcription factors (TFs), the signaling elements that relay signals into the nucleus, bind to responsive elements in promoters in a sequence-specific manner. The TFs contain DNA-binding motifs or domains. These motifs enable the proteins to bind to specific DNA sequences just as protein recognition modules enable one protein to bind to another in an amino acid sequence-specific manner. A typical promoter contains several responsive elements. This architecture allows a number of transcription factors to bind and jointly regulate transcription.
Each gene has its own promoter site consisting of the core promoter and a number of responsive elements for transcription factors. A signaling pathway that activates downstream transcription factors can regulate the

16.3 TFs Bind DNA Through Their DNA-Binding Domains 389
expression of multiple genes. The activation process is a differential one because of differences in promoter architecture from promoter site to promoter site and in the mix of proteins that are present at each. The sites can differ from one another in the mix of responsive elements, in the presence or absence of other transcription factors and coactivators (Mediator and the like), the state of the chromatin, and disposition of chromatin modifiers. This type of regulation, where multiple proteins jointly determine the regulatory outcome, is called combinatorial control.
16.3TFs Bind DNA Through Their DNA-Binding Domains
Transcription factors enter the nucleus, bind to DNA in a sequence-specific manner, and regulate gene expression. Transcription factors, like other elements of the control layer, are highly modular in design. Sequences that code distinct functions are organized into modules that can be arranged in different ways to generate a variety of functional proteins. The separable units in a typical transcription factor are
•DNA-binding domains,
•transcriptional activation domains,
•dimerization domains,
•protein-protein interaction domains, and
•nuclear localization sequences.
In the above, the protein-protein interaction domains mediate their interactions with upstream elements of the signaling pathways. The nuclear localization sequences include nuclear import sequences and/or nuclear export sequences. The DNA-binding domains are modules designed to recognize specific nucleotide sequences. Recall that the edge of each base pair is exposed on the outside of the DNA helix. The DNA-binding domains recognize distinctive patterns of hydrogen bond donors and acceptors, as well as hydrophobic patches along the edges located in the major and minor grooves. As is the case of protein-protein binding interfaces, electrostatic surface complementarity is accompanied by geometric complementarity, that is, by geometric matching of the shape of the DNA helix and protein surface. Protein-DNA binding interfaces were discussed in general terms in Chapter 4. It was noted that some DNA-binding proteins bind the major groove; others bind the minor groove; and some simultaneously contact both. Besides hydrogen bonds and hydrophobic patches, salt bridges and van der Waals contacts contribute to the establishment of good surface complementarity.
There are a number of prominent protein-DNA binding motifs. One of these is the helix-turn-helix (HTH) motif. Others include the helix-loop- helix (HLH), zinc finger, and basic region-leucine zipper (bZIP) domains.

390 16. Gene Regulation in Eukaryotes
FIGURE 16.3. Lambda repressor in a complex with DNA determined by means of X-ray crystallography: A pair of lambda repressors makes contact with the DNA double helix in its major groove. Protein helices are depicted as ribbons and DNA molecules as balls-and-sticks. The figure was prepared using Protein Explorer with atomic coordinates deposited in the Brookhaven Protein Data Bank under accession number 1LMB.
The HTH domain consists of two short alpha helices connected by a sequence of four amino acids. The first helix executes two or three turns and the second helix has four turns. The two helices are oriented 120 degrees to each other. The second helix contacts the DNA in the major groove, and the first helix assists in the positioning. HTH motifs are common in bacterial DNA-binding proteins, and are found embedded in eukaryotic homeodomains. These domains are often found in proteins needed for Drosophila development, and are widely distributed among eukaryotes from yeast to man. An example of the helix-turn-helix motif is the lambda repressor protein shown in Figure 16.3 (The lambda repressor will be discussed in Chapter 18).
The HLH motif is found in many of the transcription factors involved in eukaryotic development and regulation of adult physiological function. This motif consists of an alpha helix followed by a loop that connects the first helix to a longer second alpha helix. The loop is highly flexible and this property enables the two helices to fold and pack against one another. This ability promotes the formation of dimers. When forming a dimer a portion of the longer helix contacts the DNA, and the shorter helix contacts the corresponding shorter helix in the HLH motif of its dimerization partner. The overall result is the formation of a four-helix bundle that grips the DNA molecule.
Leucine zipper domains are present in many proteins that form dimers. These proteins consist of a pair of alpha helices. A portion of one of the helices is enriched in hydrophobic leucine residues—there is a leucine residue in every seventh amino acid position. The leucines from one protein
