
Molecular and Cellular Signaling - Martin Beckerman
.pdf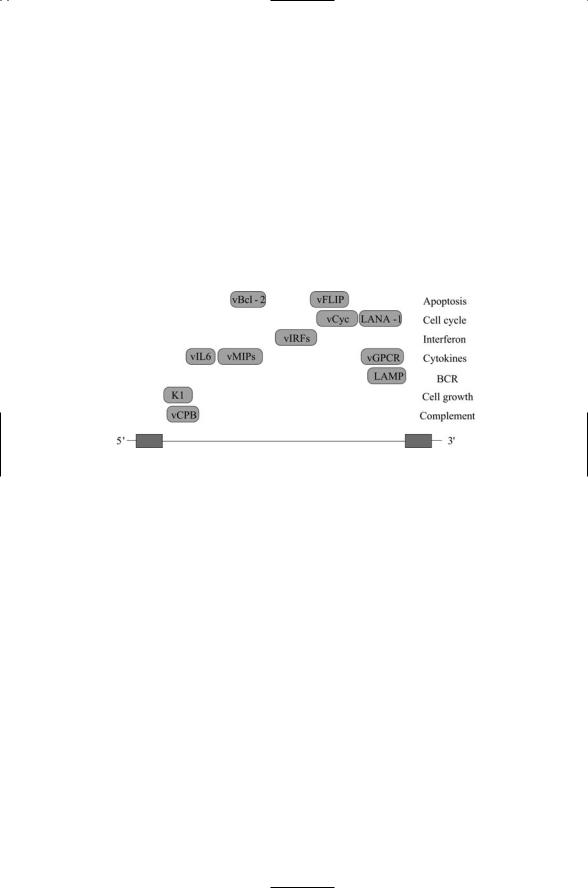
18.10 DNA and RNA Viruses that Can Cause Cancer |
451 |
||
TABLE 18.3. Tumor viruses. |
|
|
|
|
|
|
|
Virus |
Type of tumor |
|
|
|
|
|
|
DNA tumor viruses |
|
|
|
Hepatitis B virus |
Liver cancer |
|
|
Herpesviruses |
|
|
|
Epstein-Barr virus |
Burkitt’s lymphoma, |
|
|
|
nasopharylgeal carcinoma |
|
|
Kaposi’s sarcoma virus |
Kaposi’s carcinoma |
|
|
Papillomaviruses |
Cervical cancer |
|
|
RNA tumor viruses |
|
|
|
Hepatitis C virus |
Hepatocellular carcinoma |
|
|
Retroviruses |
Adult T cell leukemias |
|
|
|
|
|
|
FIGURE 18.3. Approximate locations of regulatory genes encoded in the Kaposi’s sarcoma-associated herpesvirus (KSHV) genome: The 141-kB genome is flanked by terminal repeat sequences (gray). The coding region (clear) contains two classes of regulatory genes. The first of these are the genes pirated from host genomes. The members of this group can be recognized by the presence of a letter “v” in front of familiar gene names. The second group consists of regulatory genes unique to the virus and its close relatives, such as the Epstein-Barr virus. Three examples are included in the figure: K1, latency-associated nuclear antigen type 1 (LANA-1), and latency-associated membrane protein (LAMP). Other abbreviations—Complement binding protein (CBP); macrophage inflammatory protein (MIP); interferon regulatory factor (IRF).
pesvirus (KSHV). Viruses such KSHV have pirated a large number of regulatory genes from their hosts, and through expression of these genes alter the cell cycle and growth responses of the host so that they serve the virus. They use the pirated genes, as well as genes that are unique to the viruses to degrade both the innate immune response and the adaptive immune response, and can transform cells, producing a number of cancers in humans.
Some of the most prominent regulatory genes in the KSHV genome are shown in Figure 18.3. The first group of genes depicted in the figure encodes
452 18. Regulation by Viruses
two antiapoptotic proteins. The viral Bcl-2 protein acts at the PTPC to inhibit apoptosis, and the viral FLIP acts at the DISC to throttle back death receptor signaling by preventing activation of caspase 8. The next set of gene products regulates the cell cycle so that it becomes more conducive to viral replication. The viral cyclin protein (vCyc) is similar to the cellular D cyclins and primarily binds to Cdk6. The cell cycle genes are followed in the figure by a considerable number of genes that jointly regulate immune responses and cell growth. The LAMP protein is a 12-pass transmembrane protein that inhibits signaling from the B cell receptor. The other proteins further shift the life-death signaling balance towards growth and away from apoptosis, and suppress Th1 immune responses.
18.11 HIV Is a Retrovirus
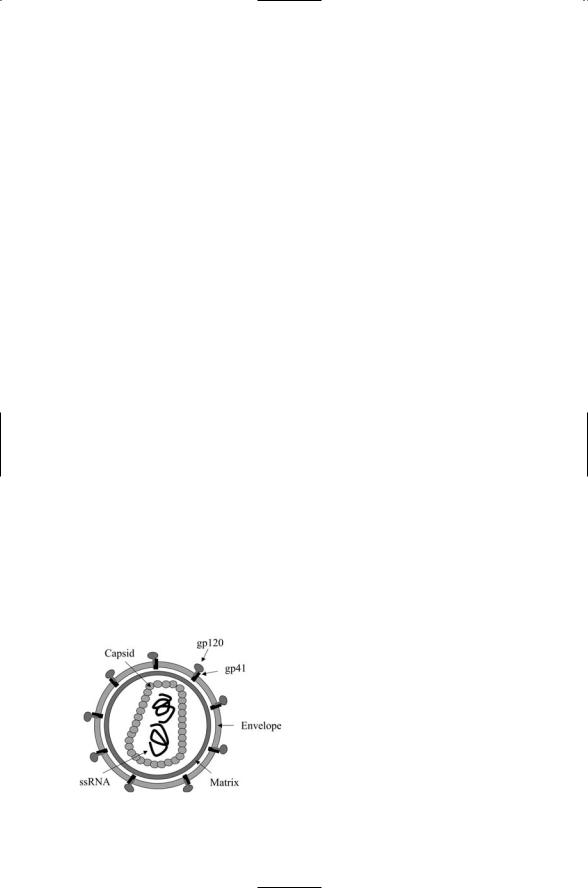
The HIV-1 virus is a member of the retrovirus family. Like others in that family the packaging of the virus is fairly elaborate. The virus capsid is encapsulated in a matrix that, in turn, is enclosed inside an envelope. Two copies of an ssRNA molecule are contained within the capsid. Each ssRNA molecule encodes 9 polygenes and 15 genes overall. The structure of the virus is depicted in Figure 18.4.
The organization of the HIV-1 genome is depicted in Figure 18.5. As was the case for the other viruses, a combination of structural and nonstructural genes is encoded. The structural genes are located on group-specific antigen (gag) and envelope (env) genes. These are proteolytically processed to yield seven protein-coding genes. The core, or CA, gene encodes the capsid protein, and the MA gene encodes the matrix protein. The third gag gene is the nucleocapsid gene, NC. It encodes a protein that associates with the ssRNA molecule. The last gag segment is the p6 gene that contains a late domain that mediates docking, budding, and release of the HIV virus at the cell surface.
The envelope polygene encodes two glycoproteins—gp120 surface (SU) and gp41 transmembrane (TM). These proteins form complexes in the
FIGURE 18.4. Structure of the human immunodeficiency virus (HIV): The virus is shown with its surface-bound gp120 and transmembrane gp41 proteins protruding out from the envelope of the virus.

18.12 Role of gp120 Envelope Protein in HIV |
453 |
FIGURE 18.5. Organization of the HIV-1 genome: Abbreviations of regulatory genes—Negative effector (nef); regulator of viral expression (rev); transactivator of viral transcription (tat); virulence infectivity factor (vif); virulence protein regulatory (vpr); virulence protein unknown (vpu).
plasma membrane. They facilitate binding and entry of the virus into the host cell. All retroviruses contain gag, env, and pol genes. The pol gene encodes enzymes required for replication of the virus. One of the enzymes is a reverse transcriptase (RT). This enzyme catalyzes the polymerization of a DNA molecule from the RNA molecule. This is a reverse, or retro, information transfer operation, hence the name “retrovirus.” The pol gene also encodes a protease (PR) that cleaves viral proteins following their translation, and an integrase (IN) that helps integrate the viral DNA into the host genome. There are six regulatory genes. These genes—vif, vpr, vpu, nef, tat, and rev—are unique to HIV and along with gp120 are responsible for HIV’s lethal behavior. These virulence-promoting genes are discussed next in greater detail.
18.12 Role of gp120 Envelope Protein in HIV
The gp120 envelope protein mediates contact with and entry into the host cell. The gp120 protein is extensively modified in the Golgi where it acquires a set of complex sugars. The glycoproteins are then transported to the cell surface where they fuse with the budding viruses and are incorporated into their envelopes. The gp120 envelope protein interacts with receptors on the cell surface, most notably with CD4 receptor on helper T cells, and uses the chemokine CCR5 receptors as coreceptors, sometimes along with the CXCR4. Binding is sequential. Binding to CD4 triggers conformational changes leading to binding to the chemokine receptors. Membrane fusion and entry into the cell follows these binding events.
Neutralizing antibodies bind to epitopes on viral surface molecules such as gp120 and block the ability of those proteins to bind receptors on host cells. The viruses are prevented from receptor-binding and entry into the cell, and are thus neutralized. In the case of HIV, most neutralizing antibodies are unable to recognize the gp120 glycoprotein. This glycoprotein avoids detection by occluding its antibody-binding sites. Antibodies bind gp120 in two places. One of these overlaps the CD4 binding site and is

454 18. Regulation by Viruses
FIGURE 18.6. Crystal structure of the HIV-1 gp120 envelope glycoprotein in a complex with CD4 and a neutralizing antibody determined by X-ray crystallography: (a) The gp120 glycoprotein is shown in a bound to the CD4 receptor expressed on helper T cells and to a Fab antibody fragment called 17b. (b) Expanded view of the gp120 core—The structure is shown rotated 90 degrees about a vertical axis from the view shown in part (a). The figure was generated using Protein Explorer with atomic coordinates deposited in the Brookhaven Protein Data Bark under accession code 1GC1.
occluded by the V1/V2 loop. The other overlaps the chemokine binding site and is occluded by the V2 and V3 loops (Figure 18.6). This is not the only technique used. A second way HIV avoids neutralizing antibodies is by conformational masking. This is a thermodynamic effect, tied to conformational changes, that allows gp120 to bind to CD4 while simultaneously avoiding neutralization by the antibodies. Yet another way used by gp120 to avoid discovery is glycosylation. A large number of changing sugar groups are added, about 20 glycans for each gp120 molecule. These act as a constantly shifting shield against neutralizing antibodies.
18.13 Early-Acting tat, rev, and nef Regulatory Genes
Prior to entry into the nucleus the HIV-1 RNA is transcribed into double stranded DNA by the reverse transcriptase (RT). A preinitiation complex (PIC) is formed consisting of the viral DNA, integrase (IN), multiple matrix
(MA) protein copies, nucleocapsid protein (NC), p6, and Vpr. The PIC binds to the pore and is then sent into the nucleus using NLSs embedded in viral and associated cellular proteins.
The 9-kB HIV genome encodes 15 proteins, but nascent transcripts are spliced in about 30 different ways to produce a variety of messenger RNA

18.13 Early-Acting tat, rev, and nef Regulatory Genes |
455 |
FIGURE 18.7. Regulation of transcription elongation by Tat: A portion of the HIV promoter is shown highlighting the upstream NF-kB and Sp1 binding sites, and TATA box. The RNAP II is depicted with several of its cofactors transcribing the DNA into an RNA chain. A portion of the RNA chain (shown in black), the TAR, is bound by Tat which then recruits the Cdk9/Cyclin T1 complex. The Cdk9 kinase hyperphosphorylates the C-terminal domain (CTD) of the RNAP II molecule.
molecules. In the early stages of HIV infection, short 2-kB mRNAs are produced and exported from the nucleus. These short transcripts encode Tat, Rev, and Nef proteins, and the genes for these are referred to as early acting genes. Later in the infection cycle longer 4-kB variants and full length mRNAs are made, and the genes transcribed at that time are termed late acting genes.
The HIV virus not only supplies its own promoter, as do other retroviruses, but also supplies its own transcription activator, Tat. The Tat protein is an RNA binding protein. It binds to a segment of nascent HIV RNA chain called the transactivating response (TAR) region located at the 5¢ end. A number of cellular proteins recognize the TAR sequence and are recruited to the site. As depicted in Figure 18.7, these include Cyclin T1 and its cyclin-dependent kinase Cdk9, which hyperphosphorylates the C- terminal domain of RNAP II. In the absence of hyperphosphorylation, the transcripts generated by RNAP II tend to be short. Hyperphosphorylation prevents premature elongation termination and enables the enzyme to catalyze full length transcripts.
Rev is a sequence-specific RNA binding protein that binds to a region called the Rev responsive region (RRE) located in env gene transcripts. The Rev protein contains a nuclear export sequence (NES) and mediates the export of all transcripts except those of tat, rev, and nef from the nucleus into the cytoplasm, where they are translated into the virus components. In the early stages of an HIV infection, before Rev levels are sufficient to support its export function, transcripts longer than 2 kB that get made collect in the nucleus. In the later stages of HIV infection, Rev facilitates the export of longer and full length transcripts from the nucleus.
The Nef protein helps the virus to control signaling by its host and creates an environment conducive to replication of the virus. One of the key targets

456 18. Regulation by Viruses
of Nef is the MHC-I complex on the cell surface. Recall that MHC-I complexes are used to present antigens derived from, for example, viruses, on the surface where they can be recognized by killer cells of the immune system. Nef stimulates the internalization of these receptors and directs them to the Golgi. Another target of the Nef protein is the CD4 receptor. These are internalized and sent to digestive compartments. The MHC-I and CD4 receptors are not the only immune system signaling elements targeted by Nef. It also downmodulates the CD28 coreceptor. All of these actions help disable signaling between cells of the immune system, enabling infected cells to evade detection and destruction. In macrophages, which along with CD4 T cells are the main targets of HIV, Nef induces the release of chemokines that attract resting T cells and help to transmit the virus to uninfected cells.
18.14Late-Acting vpr, vif, vpu, and vpx Regulatory Genes
Viral protein U (Vpu) is found exclusively in HIV-1, while viral protein X (Vpx) is restricted to HIV-2 and simian immunodeficiency virus (SIV). Vpu assists in the degradation of CD4 in the endoplasmic reticulum and in the exit of the virus from the cell. It binds CD4 in the ER and targets it for degradation in the ubiquitin-proteosome pathway thereby preventing its translocation to the plasma membrane.
Viral protein R (Vpr) encoded by the vpr gene, is a small protein, containing just 96 amino acids. It is soluble and is able to pass through membranes. Vpr acts in several ways to increase HIV survival in cells that it infects. It assists in the nuclear localization of the PIC, and triggers arrest of the cell cycle at G2/M transition by blocking Cdk1/Cyclin B complex formation that drives the cell into mitosis. The Vpr protein acts as a modulator of apoptosis. It migrates to the mitochondria where it works together with proteins resident in the PTPC to shift the balance either towards or away from apoptosis. It can work with the antiapoptotic Bcl-2 protein to inhibit apoptosis and work at the ANT and VDAC to increase mitochondrial membrane permeability and promote apoptosis.
Viral infectivity factor (Vif) counters the antiviral activities of a cellular agent with a long name—apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G, or APOBEC3G. While the retroviral RNA is being reverse-transcribed into DNA this enzyme catalyzes the conversion of “C” nucleotides to “U” nucleotides. These alterations prevent replication of retroviruses in cells, referred to as being nonpermissive, that express the APOBEC3G enzyme. The Vif protein renders this antiviral enzyme ineffective by binding to it and speeding up its degradation in the ubiquitinproteosome pathway.
18.15 Bacteriophages’ Two Lifestyles: Lytic and Lysogenic |
457 |
18.15Bacteriophages’ Two Lifestyles: Lytic and Lysogenic
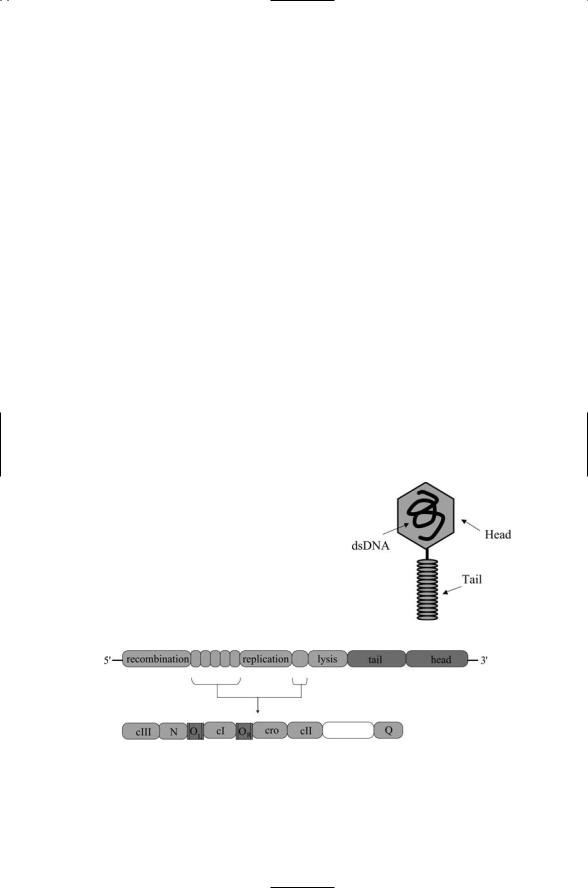
Bacteriophages, or phages, are bacteria-infecting viruses. Bacteriophage lambda is perhaps the best studied of this group of viruses. Like many others it has two alternative lifecycles—lytic and lysogenic. In its lytic cycle, the virus attaches to its host, and its nucleic acids—dsDNA—enter the cell while the head and tail sections of the capsid (Figure 18.8) remain outside.
The DNA is rapidly replicated and capsid proteins are synthesized. The virus components are reassembled and the resulting virions are released from the host cell through lysis, in which the cell membrane ruptures, and the viruses escape leaving behind a dying cell.
In its lysogenic cycle, the phage DNA is integrated into the host cell DNA rather than being immediately replicated. It becomes a prophage, replicating as part of the bacterial host’s chromosome. It continues to do so until environmental conditions are such that a lytic cycle is preferable. When those conditions arise it switches from the lysogenic cycle to the lytic cycle. It rapidly replicates itself and lyses the cell.
The organization of the bacteriophage lambda genome is depicted in Figure 18.9. Several sets of nonstructural genes are shown. These genes encode enzymes required for recombination (integration of the viral genome into the host’s chromosome), replication, and lysis. Six regulatory genes are interspersed among the aforementioned nonstructural genes.
FIGURE 18.8. Bacteriophage lambda: The capsid of the bacteriophage is organized into a head section, which contains the DNA, and a tail, which assists in attachment of virus to its host and injection of the viral DNA into the bacterial host.
FIGURE 18.9. Organization of the bacteriophage lambda genome and its regulatory genes: The organization of the six regulatory genes and two noncoding operator regions are depicted in the lower portion of the figure.

458 18. Regulation by Viruses
Their gene products control the decision between the lytic and lysogenic life.
18.16 Deciding Between Lytic and Lysogenic Lifestyles
The regulatory network used by the bacteriophage lambda to switch between lysis and lysogeny is one of the best characterized genetic circuits.
This genetic network consists of six regulatory genes (N, Q, cI, cro, cII, and cIII), three promoters (PL, PR, and PcI), and two noncoding operator regions (OL and OR). The cI gene is called the lambda repressor because when it is expressed its gene product, the CI protein, represses transcription of the genes needed for viral replication thereby favoring the lysogenic lifestyle. For the same reason the Cro protein is called the lambda counterrepressor because when it is expressed it facilitates transcription of the genes needed for the lytic lifestyle. The decision process is represented schematically in Figure 18.10.
PL and PR are left and right promoters. PL is responsible for transcribing the left hand portion of the network that includes cIII and N. PR is responsible for transcribing the right hand portion that includes cro, cII and Q. OL and OR are noncoding operator regions each consisting of three cooperative binding sites. cI is the lambda repressor gene, which, when bound to the operator sites, shuts down transcription of all lambda genes except that of the repressor itself. It has its own promoters, PRE and PRM. These have been represented in Figure 18.10 as PcI. When cI is expressed it binds to
FIGURE 18.10. Operation of the phage lambda decision circuit: The central portion of the regulatory circuit in shown in an expanded view in the lower portion of the diagram. The lysogenic state is selected when CI binds to the operator sites and represses transcription from the left and right promoters. The lytic state is chosen when the Cro protein binds to the operator sites.

18.17 Encoding of Shiga Toxin in E. coli |
459 |
sites nearest the left and right promoters, thereby blocking transcription of the other genes, and the phage remains a prophage. The cro protein product is an antirepressor that shuts off cI when it is bound to the operator sites. As shown in Figure 18.10, it favors the innermost sites in the left and right promoters. When it binds to these sites it shuts off cI transcription while allowing transcription of the other genes from the left and right promoters. This circuit exploits both positive and negative feedback.
One of the most interesting aspects of the phage decision circuit is the occurrence of cooperativity in the two operator regions. Binding of a protein to a site in one of the operator regions increases the probability that a second protein will bind to a nearby site that would otherwise be unoccupied due to weak binding. The initial binding events thus serve to recruit additional proteins to the regulatory regions where they jointly control transcription. This type of control is not unique to bacteria or to phages; it was encountered earlier in the chapter on eukaryotic gene regulation.
The decision circuit operates under environmental control. The decision circuit is sensitive to good versus poor growth conditions and to exposure to ultraviolet radiation. The outcome of the race between lysogeny and lysis is determined by the concentration of CII in the cell. These proteins act as enhancers of cI transcription. The concentration of CII in a healthy cell is usually maintained at a low level by the activity of host proteases that are sensitive to glucose levels. When nutrients are plentiful, CII levels are low resulting in a stable lytic state of replication and release of new phages. The CII concentration under poor bacterial growth conditions is elevated leading to CI levels that are high enough to send the phage into a lysogenic state.
The phage can transition from lysogeny to lysis in response to DNA damage arising from UV or ionizing radiation. RecA proteins are synthesized by the host in response to ultraviolet damage to the cellular DNA.
These proteins stimulate the repair of damaged DNA by inactivating the LexA repressor. The LexA repressor is similar to the lambda repressor CI. When phage lambda is present, RecA targets CI as well. There is an increased expression of Cro proteins leading to a lytic stage where the virus escapes from the UV damaged cell.
18.17 Encoding of Shiga Toxin in E. coli
Shiga toxin genes are encoded by a lambda-like phage in enterohaemorrhegic Escherichia coli. In the last chapter, it was noted that harmless strains of bacteria such as the harmless K12 variety of E. coli differ from their pathogenic relatives in that the latter contain additional genes acquired mostly through horizontal gene transfer. Bacteriophages are a common means of transfer of DNA from one bacterial species to another. The Shiga

460 18. Regulation by Viruses
FIGURE 18.11. Phage genes incorporated into the genomes of several strains of enterohaemorrhagic E. coli: The toxin genes StxA and StxB are situated in between the replication and late antitermination (Q) genes on the left and lysis genes on the right.
toxin genes that render strains of E. coli such as O157 : H7 so harmful provide an excellent example of this mechanism. As shown in Figure 18.11 the Shiga toxin genes StxA and StxB genes are encoded in E. coli O157 : H7 in a stretch of DNA almost identical to that of bacteriophage lambda. The gene sequence depicted in the figure is found not only in the O157 : H7 strain but also in the several other E. coli and Shigella dysenteriae strains.
Shiga toxin genes are composed of catalytic A subunits and glycolipidbinding B subunits. They are regulated by factors intrinsic to the bacterial genome and by the bacteriophage life cycle. There is a binding site in the operator region upstream of the A gene subunit for the iron binding Fur protein. When iron is plentiful the protein is able to bind to the operator and block transcription. This repression is relived in low-iron environments. Production of toxins is also increased when the bacteriophage replicates and is released from the host cell through lysis. This places the genes for the toxins under the control of environmental factors such as increased H2O2 levels, which can damage DNA, and the presence of antibiotics.
References
General Reading
Schaechter M, Engelberg NC, Eisenstein BI, and Medoff G [1999]. Mechanisms of Microbial Diseases (3rd edition). Baltimore: Lippincott, Williams and Wilkins.
Walker TS [1998]. Microbiology. Philadelphia: W.B. Saunders and Company.
Viral Entry into the Cell
Greber UF [2002]. Signalling in viral entry. Cell. Mol. Life Sci., 59: 608–626. Overbaugh J, Miller AD, and Eiden MV [2001]. Receptors and entry cofactors for
retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphatidylinositol-anchored and secreted proteins.
Micro. Mol. Biol. Rev., 65: 371–389.
Sieczkarski SB, and Whittaker GR [2002]. Dissecting virus entry via endocytosis. J. Gen. Virol., 83: 1535–1545.
Virus Cytoplasmic Transport
McDonald D, et al. [2002]. Visualization of the intracellular behavior of HIV in living cells. J. Cell Biol., 159: 441–452.
Seisenberger G, et al. [2001]. Real-time single-molecule imaging of the infection pathway of an adeno-associated virus. Science, 294: 1929–1932.
