
Molecular and Cellular Signaling - Martin Beckerman
.pdf
17.17 Pathogenic Species Possess Virulence Cassettes |
431 |
of these is conjugation, in which two cells establish direct contact with one another. Sex pili are formed and genetic material in the form of a plasmid is sent from the donor bacterium to the recipient. This is perhaps the most common way of carrying out horizontal gene transfer. A second way of transferring genetic material is through transduction, where a bacteriophage picks up genetic material from one bacterium and delivers it to another. A third way of acquitting genetic material is through transformation, in which a bacterium becomes competent to capture exogenous DNA from its local environment as discussed in the previous section.
Resistance to antibiotics can be spread rapidly through horizontal gene transfer. One consequence of horizontal genetic transfer is that groups of genes that endow one bacterial species with antibiotic resistance can be transferred in minutes to hours to another species.The transfer of genetic material, that is, the sharing of blueprints for making useful proteins among bacteria, is a particularly potent adaptive response. It is far more efficient to simply transfer a complete set of genes than to have each species “invent” for itself the genes one by one by means of random mutations. In examining the complete genome sequences of these pathogens it becomes clear that entire regions of DNA have been acquired from other species in the past.
Antibiotics are agents synthesized by fungi and bacteria that kill competing microbes. There are several different kinds of antibiotics. Some antibiotics act on bacteria by causing damage to their cell walls. Other antibiotics impede the ability of the bacteria to carry out protein synthesis or to disrupt the replication of DNA or to interefere with metabolism. Peni- cillin-type antibiotics such as Ampicillin and Methicillin, cephalosporin antibiotics, and Vancomycin attack bacterial cells walls. Protein synthesis impeding antibiotics include tetracycline, aureomycin, streptomycin, and Erythromycin. Quinolones disrupt DNA replication, and sulfur drugs interfere with bacterial metabolism.
There are several ways of conferring resistance to antibiotics. One way is to synthesize biomolecules that destroys the antibiotics. Another approach is to pump the antibiotics rapidly out of the cell thereby keeping its intracellular concentration so low that it is ineffective. Still another way of dealing with the antibiotic is to synthesize cellular agents that bind antibiotics and neutralize them thereby acting as intracellular blockers.
17.17 Pathogenic Species Possess Virulence Cassettes
Most bacterial strains are harmless, but some can be deadly. In pathogenic strains additional proteins such as toxins and fimbriae components are typically encoded on plasmids and other mobile genetic elements that can be readily transferred and exchanged between species. It is these additional proteins that endow bacteria with their disease-causing capabilities. For example, the standard laboratory strain of Escherichia coli K12 is harmless.

432 17. Cell Regulation in Bacteria
Its genome is 4.6 MBp long, while the pathogenic strain of E. coli O157 is considerably larger—5.4 MBp in length. In E. coli O157 there are “O” islands and “K” islands, clusters of genes that encode a total of 1387 proteins not found in the K12 strain. The additional proteins encoded by the E. coli O157 strain endow the bacterium with enterohaemorrhagic abilities.
One of the most striking findings from sequencing complete genomes of microbes is that bacterial genomes are riddled with phage genes. It appears that bacteriophages are a major source of mobile genetic material moving in and out of bacteria. In many species, pathogenicity and virulence of the bacterial strain are tied to the presence of a prophage, the inactive (lysogenic) bacteriophage whose genes have been integrated into the bacterial genome. An example of this coupling is provided by group A Streptococcus (GAS) strains. These bacteria promote sore throats, impetigo, acute rheumatic fever (the major heart disease of children), necrotizing fasciitis (the disease caused by flesh-eating bacteria), and toxic shock syndrome. The various strains (serotypes) of GAS are given “M” designations according to differences in a family of streptococcus cell surface proteins called M proteins, which impede the engulfment by phagocytes. The M18 strain, responsible for acute rheumatic fever contains phage genes that encode the disease-causing toxins. Another strain, M3, which produces toxic shock syndrome and necrotizing fasciitis, has a different set of phage genes from the M18 strain, and the strain most often responsible for sore throats, M1, has yet another set of phage genes.
Pathogenicity islands, or virulence cassettes, are clusters of functionally related virulence genes that are absent in nonpathogenic organisms. The term “virulence” denotes the ability to cause disease. A virulence factor is the designation given to an individual disease-causing or disease-promot- ing agent of an organism. Some examples of virulence factors are toxins, surface adhesion molecules, control molecules, and virulence regulatory proteins (transcription factors). Virulence cassettes, consisting of ensembles of these proteins, may be located on plasmids or on other mobile genetic elements that can be transferred from one bacterium to another. They may be found on the bacterial chromosome, as well. In all cases they encode a complete suite of proteins needed to promote virulence.
The virulence cassettes of enteropathogenic E. coli (EPEC) strains allow them to build their own signal transduction pathway in a host cell, which they use to remodel the host’s cytoskeleton. The bacteria first send soluble proteins into the host cell membrane. The proteins are then modified to form physiologically functional receptors for its bacterial ligands, thereby establishing a firm contact between cells. Upon appropriating the communication machinery the bacterium uses the host’s resources to erect a pedestal on the host cell’s surface. This pedestal supports the subsequent assembly of a bacterial colony.
Virulence cassettes are used to transfer agents from bacterium to host. The most common forms, known as Type III and IV secretion systems, are
17.18 Bacterial Death Modules |
433 |
encountered in many pathogenic bacterial species. They are similar enough to one another to be interchangeable and to have been acquired by horizontal transfer from species to species. The best characterized system of this sort is the Yop virulon, a Type III system encoded on a plasmid. It contains
(i) a secretion apparatus, (ii) a delivery system that sends bacterial proteins into the eukaryotic host, (iii) a control unit, and (iv) an agent effector module that disarms the host signal transduction network. The objective of Yersinia is to survive and replicate in the lymphoid tissue of its host. The prime danger to survival is from phagocytes produced by the host organism’s immune system. The virulons inactivate the phagocytes by activating the phagocytes’ apoptosis (suicide) circuitry. Yersinia avoids attracting the attention of the immune system. The secretion apparatus and delivery system is activated by cell-to-cell contact and inserts agents into the target in a highly directional way so that there is little or no leakage into the intercellular spaces.
17.18 Bacterial Death Modules
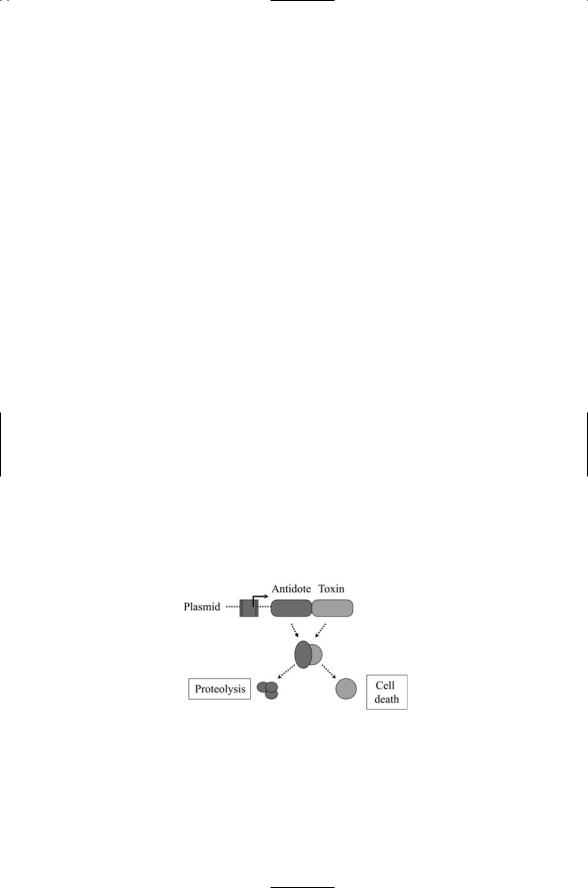
Bacteria employ death modules to kill competing bacteria and in some instances to commit suicide. Bacteria are able to synthesize and release toxins without themselves being harmed. To accomplish this feat the bacteria synthesize the antidote to the toxin at the same time that they make the toxin. The genes for both toxin and antidote, or antitoxin, usually reside on a plasmid and are transcribed from the same promoter (Figure 17.11). The toxin is long-lived while the antidote is unstable and short-lived. As long as the plasmid is present and active both toxin and antidote get made and the bacterium is protected against the former.
Toxin-antidote pairings can serve as the core element in suicide modules.
As is the case for self-protection, a long-lived toxin is paired with a short-
FIGURE 17.11. Bacterial death modules: The genes for toxin and antitoxin (antidote) reside side by side on a plasmid. When the genes are expressed the antidote binds the toxin and prevent it from binding to its substrate. In response to suicide signals, proteolytic enzymes degrade the antidote; the toxin then binds to its substrate leading to cell death.

434 17. Cell Regulation in Bacteria
lived antidote that antagonizes the activity of the toxin. If the plasmid is disabled the antidote will not be made, and because it has a short half-life the antidote will decay away rapidly. When the antidote is removed either by loss of the plasmid or by some other means such as proteolytic cleavage, the toxin is free to degrade its substrate, usually an enzyme critical for cellular survival, and the host cell dies. It forming colonies, bacteria sometimes use death modules of this type to selectively remove a potion of their population. This may be viewed as a bacterial form of programmed cell death.
17.19Myxobacteria Exhibit Two Distinct Forms of Social Behavior
Myxobacteria are gram-negative, aerobic soil bacteria that feed on decomposing vegetation. When nutrient supplies are plentiful species such as M. xanthus form feeding swarms that glide together over solid surfaces and prey on other bacteria. The members of a swarm are free to come and go, explore other feeding sites, and establish colonies. This type of social behavior changes when nutrients are no longer available. Under starvation conditions the bacteria organize into a differentiated hemispherical structure called a fruiting body. Rod cells inside the fruiting body differentiate into spores that are subsequently dispersed. When these spores encounter a hospitable environment they reestablish a feeding colony of gliding bacteria.
The transition from feeding swarm to fruiting body is initiated by starvation signaling. When bacteria are deprived of essential nutrients they reduce or halt protein synthesis, DNA replication, and cell division and enter a stationary phase. Any such alteration in cellular physiology is referred to as a stringent response. One of the main elements of the stringent response is the accumulation of the highly phosphorylated, guanosine (penta)tetraphosphate in the cell. In bacteria, RelA (a synthase) and SpoT (a hydrolase) synthesize pppGpp and ppGpp from GTP and GDP using ATP as a donor. These nucleotides serve as intracellular second messengers of starvation conditions. Under adequate nutrient conditions these molecules are maintained in the bacterial cell at low, steady state concentrations. Starvation conditions, in the case of M. xanthus, a lack of amino acids or carbon compounds supplied by prey, stimulate an increased ppGpp production by RelA. The ppGpp molecules bind and alter the activity of RNA polymerase shifting the global pattern of gene expression from growth to a stationary phase. In Streptomyces coelicolor, the stringent response triggers an increase in antibiotic production and morphological differentiation. In M. xanthus, the buildup in ppGpp upregulates several asg genes, one result of which is to release proteases that generate a mixture of amino acids and peptides collectively referred to as A-factors. The A-factors are secreted

17.20 Structure Formation by Heterocystous Cyanobacteria |
435 |
from the myxobacteria and act as quorum-sensing signals. If the concentration of A-factors is adequate, fruiting body formation proceeds.
A-signaling in M. xanthus is followed by C-signaling that determines which cells in the nascent fruiting body are fated to become spores and which cells will retain their original rod shape. Transmission of C-signals occurs between cells that are in close, end-to-end contact. Transmission is ineffective when neighboring cells lie side by side. The circuitry involved in C-signaling closely resembles that used in Notch signaling in eukaryotes. C- signals transmitted between ends of cells activate FruA, a transcription factor that stimulates the expression of the csgA gene that encodes the C- signals. This positive feedback loop increases FruA activity above thresholds for its activation of frz genes and then dev genes. The frz genes encode proteins that mediate the aggregation of the cells into mounds, and the dev genes encode proteins that promote the differentiation of rod cells into spores. Because of the geometric constraint on C-signaling, cells in the periphery never experience threshold-exceeding concentrations of FruA while those in the nascent fruiting body reach critical signal densities.
17.20Structure Formation by Heterocystous Cyanobacteria
Heterocystous cyanobacteria form differentiated structures with properties usually associated with multicellular eukaryotes. Cyanobacteria are phototrophs and, like plants, are capable of oxygenic photosynthesis in which CO2 is removed and O2 is produced. These organisms have minimal requirements for survival—water, light, CO2, and some salts that supply N2, iron, phosphorus, and sulfur. For many years the cyanobacteria were referred to as “blue-green algae,” but are now recognized as being Gram-negative bacteria. Ancestral cyanobacteria first appeared some 3.5 billion years ago and are believed to be responsible for the oxygenation of the Earth’s atmosphere. Photosynthesis in cyanobacteria is carried out in thylakoid membranes in the same way as it is done in plant cells. Similarities in size and structure, the presence of chlorophyll A, photosystems I and II, and similarities in control elements provide evidence that present day chloroplasts and cyanobacteria share a common ancestry.
Cyanobacteria are an essential part of the world’s ecology. Not only do they release oxygen but they also fix nitrogen (and carbon). Most organisms cannot make use of atmospheric nitrogen. Cyanobacteria are able to fix nitrogen, that is, they can convert atmospheric nitrogen into inorganic nitrogen that can be used for biological purposes by other organisms. They are a main component of marine plankton, the dense collection of (mostly) small drifting organisms that cover the world’s oceans and produce 90% of the planet’s oxygen. They are also found in large numbers in the surface regions of lakes, are a major component of communities known as micro-

436 17. Cell Regulation in Bacteria
bial mats found in the hot springs of Yellowstone National Park, and populate nearly all ecosystems.
Members of genera such as Calothrix and Anabaena can form differentiated structures. The structures take the form of long filaments in which nitrogen-fixing heterocysts are interspersed at regular intervals among photosynthetic vegetative cells. The heterocysts reduce N2 to ammonia but do not carry out photosynthesis or carbon fixation. The vegetative cells, on the other hand, perform photosynthesis and fix carbon. The technical problem being solved through multicellularity is the need to separate photosynthesis from the nitrogen-fixing due to the inactivation by oxygen of the nitrogenase complex responsible for nitrogen-fixing. The heterocysts possess a protective envelope and turn off their photosystem II. They export inorganic nitrogen to their neighbors and import nutrients. About 1 in 10 to 20 cells in a filament is a heterocyst. Cell-to-cell signaling is used to establish and maintain the pattern of cell differentiation just as it does in multicellular eukaryotes. Most strikingly, the heterocysts are terminally differentiated cells, like those in metazoan tissues.
17.21Rhizobia Communicate and Form Symbiotic Associations with Legumes
Rhizobia is the name given collectively to several genera of rod-shaped,gram- negative bacteria that fix nitrogen and make it available for use by legumes. In poor soils they provide a major source of fixed nitrogen to grain legumes such as soybeans, peas, and beans, as well as to forage legumes such as alfalfa and clover. Rhizobia are free-living soil bacteria that enter and colonize plant root cells. They stimulate the formation of nodules, specialized root organs, and in the nodules they convert atmospheric nitrogen into ammonia and export it to their hosts, and receive carbon compounds in exchange.
The process whereby the bacteria and host plant establish their symbiotic association involves several stages of signaling between bacteria and plant root, rounds of gene expression, and differentiation and cellular/tissue remodeling. To initiate the process, the plant roots exude signaling compounds called flavinoids that attract the bacteria to the root surface. The rhizobia attach to young root hairs and signal the root cells by secreting glycolipids called nod factors. In response to nod factors, the plant cells starts to remodel their actin and microtubule cytoskeletons and form nodules. First, the bacteria become entrapped between cell walls of stimulated (deformed) root hairs, and the plant cell walls undergo hydrolysis resulting in lesions. New cell wall material is then deposited resulting in the formation of a tubule called an infection thread that is filled with rhizobia. The infection threads are the doorways to the root cells. The infection thread network expands into the root cortex while the bacteria multiply. Eventually, the bacteria exit the network of threads and enter the cytoplasm

References and Further Reading |
437 |
of the root cells. A nodule is formed containing a plant cell-derived peribacteroid membrane. This membrane envelops the bacteria, which multiply and differentiate into bacteroids. In some symbiotic associations the bacteroids differ greatly in morphology compared to their free-living forms, becoming several times larger and Y-shaped in form. Within the nodules the bacteroids and legumes exchange metabolites, which pass through pores and channels in the peribacteroid and bacteroid membranes.
Initial plant bacteria-signaling events not only launch the nodule-forming process but also confer specificity and filter out potentially harmful associations from helpful symbiotic ones. Flavinoids secreted by the plants bind to NodD proteins situated in the cytoplasmic membrane of the bacteria. The NodD proteins are members of the LysR family of transcription factors.They serve as sensors of plant signals and as activators of genes whose promoters contain a 49 Bp sequence called a nod box. Binding by favinoid-NodD complexes results in the activation of the NodA, NodB, and NodC genes common to all rhizobia. They encode enzymes that catalyze the synthesis of the lipooligosaccharide core, or backbone, of the nod factors. Structural nod genes that are species-specific are expressed at the same time. These genes encode enzymes that modify or decorate the nod factor backbones in a manner that depends upon the structure of the flavinoids.
References and Further Reading
Gilmore MS, and Ferretti JJ [2003]. The thin line between gut commensal and pathogen. Science, 299: 1999–2002.
Whitman WB [1998]. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA, 95: 6578–6583.
Xu J, and Gordon JI [2003]. Honor thy symbionts. Proc. Natl. Acad. Sci. USA, 100: 10452–10459.
Gene Organization
Busby S, and Ebright RH [1994]. Promoter structure, promoter recognition and transcription activation in prokaryotes. Cell, 79: 743–746.
Ptashne M, and Gann A [1997]. Transcription activation by recruitment. Nature, 386: 569–577.
Sigma Factors
Cannon WV, Gallegos MT, and Buck M [2000]. Isomerization of a binary sigma-pro- moter DNA complex by transcription activators. Nature Struct. Biol., 7: 594–601.
Gralla JD [2000]. Signaling through sigma. Nature Struct. Biol., 7: 530–532.
Sharp MM [1999]. The interface of s with core RNA polymerase is extensive, conserved, and functionally specialized. Genes Dev., 13: 3015–3026.
Flagellar Assembly
Aldridge P, and Hughes KT [2002]. Regulation of flagellar assembly. Curr. Opin. Microbiol., 5: 160–165.

438 17. Cell Regulation in Bacteria
Chilcott GS, and Hughes KT [2000]. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica, Servovar typhimurium. and Escherichia coli. Micro. Mol. Biol. Rev., 64: 694–708.
Kalir S, et al. [2001]. Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science, 292: 2080–2083.
Sporulation
Burbulys D, Trach KA, and Hoch JA [1991]. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell, 64: 545–552.
Fabret C, Feher VA, and Hoch JA [1999]. Two-component signal transduction in Bacillus subtilis: How one organism sees its world. J. Bacterial., 181: 1975–1983.
Jiang M, et al. [2000]. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol. Microbiol., 38: 535–542.
Perego, M, Glaser P, and Hoch JA [1996]. Aspartyl-phosphate phosphatases deactivate the response regulator components of the sporulation signal transduction system in Bacillus subtilis. Mol. Microbiol., 19: 1151–1157.
Cell Differentiation in Caulobacter
Domain IJ, Quon KC, and Shapiro L [1997]. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell, 90: 415–424.
Jenal U [2000]. Signal transduction mechanisms in Caulobacter crescentus development and cell cycle control. FEMS Microbiol. Revs., 24: 177–191.
Laub MT, et al. [2000]. Global analysis of the genetic network controlling a bacterial cell cycle. Science, 290: 2144–2148.
Quon KC, et al. [1998]. Negative control of bacterial DNA replication by a cell cycle regulatory protein that binds at the chromosome origin. Proc. Natl. Acad. Sci. USA, 95: 120–125.
Shapiro L, McAdams HH, and Losick R [2002]. Generating and exploiting polarity in bacteria. Science, 298: 1942–1946.
Antigenic Variation
Barbour AG, and Restrepo BL [2000]. Antigenic variation in vector-borne pathogens. Emer. Infect. Dis., 6: 449–457.
Quorum Sensing
Davies DG, et al. [1998]. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science, 280: 295–298.
Fuqua WC, Winans SC, and Greenberg EP [1994]. Quorum sensing in bacteria: The LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol., 176: 269–275.
Kleerbezem M, et al. [1997]. Quorum sensing by peptide pheromones and two-com- ponent signal transduction systems in Gram-positive bacteria. Mol. Miocrbiol., 24: 895–904.
Schauder S, and Bassler BL [2001]. The language of bacteria. Genes Dev., 15: 1468–1480.
Van Delden C, and Iglewski BH [1998]. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emer. Infect. Dis., 4: 551–560.

Problems 439
Biofilms and Disease
Anderson GG, et al. [2003]. Intracellular bacterial biofilm-like pods in urinary tract infections. Science, 301: 105–107.
Costerton JW, Stewart PS, and Greenberg EP [1999]. Bacterial biofilms: A common cause of persistent infections. Science, 284: 1318–1322.
Singh PK, et al. [2000]. Quorum sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature, 407: 762–764.
Horizontal Gene Transfer, Antibiotic Resistance, and Bacteriophages
Casjens S [2003]. Prophages and bacterial genomics: what have we learned so far?
Mol. Microbiol., 49: 277–300.
Frankel G, et al. [1998]. Enteropathogenic and enterohaemorrhagic Escherichia coli: More subversive elements. Mol. Microbiol., 30: 911–921.
Hacker J, Hentschel U, and Dobrindt U [2003]. Prokaryotic chromosomes and disease. Science, 301: 790–793.
Karaolis DKR, et al. [1999]. A bacteriophage encoding a pathogenicity island, a type-IV pilus and a phage receptor in cholera bacteria. Nature, 399: 375–379.
Koch AL [2003]. Bacterial walls as target for attack: Past, present and future research. Clin. Microbiol. Rev., 16: 673–687.
Lowy FD [2003]. Antimicrobial resistance: The example of Staphylococcus aureus. J. Clin. Invest., 111: 1265–1273.
Ochman H, Lawrence JG, and Groisman EA [2000]. Lateral gene transfer and the nature of bacterial innovation. Nature, 405: 299–304.
Wagner PL, and Waldor MK [2002]. Bacteriophage control of bacterial virulence. Infect. Immun., 70: 3985–3993.
Virulence Cassettes and Factors
Aizenman E, Engelberg-Kulka H, and Glaser G [1996]. An Escherichia coli chromosomal “addiction module” regulated by 3¢,5¢-bispyrophosphate: A model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA, 93: 6059–6063.
Cornelis GR, and Wolf-Watz H [1997]. The Yersinia Yop virulon: A bacterial system for subverting eukaryotic cells. Mol. Microbiol., 23: 861–867.
Groisman EA, and Ochman H [1996]. Pathogenicity islands: Bacterial evolution in quantum leaps. Cell, 87: 791–794.
Death Modules and Programmed Cell Death
González-Pastor JE, Hobbs EC, and Losick R [2003]. Cannibalism by sporulating bacteria. Science, 301: 510–513.
Webb JS, et al. [2003]. Cell death in Pseudomonas aeruginosa biofilm development. J. Bacteriol., 185: 4585–4592.
Problems
17.1Nonfilamentous cyanobacteria solve the problem of separating photosynthesis from nitrogen-fixing in a way different from heterocystous cyanobacteria. The nonfilamentous forms carry out photosynthesis during the day and fix nitrogen at night. The separation of metabolic

440 17. Cell Regulation in Bacteria
tasks is made possible by the presence of an internal clock that synchronizes the organism’s metabolic processes with Earth’s 24-hour rotation (circadian) period.
Circadian clocks are found in plants where they regulate the opening and closing of plant leaves with respect to the daily cycle of light and dark. They are found in protists, fungi, and animals, as well. All share a common set of properties such as the use of feedback loops and the introduction of time delays. How might a circadian clock be set up in a bacterium? How might the mechanism(s) used to fix the period and phase differ from those employed by eukaryotes?
Suggested Reading
Dunlap JC [1999]. Molecular bases for circadian clocks. Cell, 96: 271–290.
Ishiura M, et al. [1998]. Expression of a gene cluster KaiABC as a circadian feedback process in cyanobacteria. Science, 281: 1519–1523.
Xu Y, Mori T, and Johnson CH [2003]. Cyanobacterial circadian clockwork: Roles of KaiA, KaiB and the KaiBC promoter in regulating KaiC. EMBO J., 22: 2117–2126.
Young MW, and Kay SA [2001]. Time zones: A comparative genetics of circadian clocks. Nature Rev. Genet., 2: 702–715.
17.2Some bacteria swim from place to place using a rotating flagellum at the back end to provide a driving force in the fluid medium. Swimming is not the only method exploited by bacteria for motility. Another means of locomotion is gliding motility. Social bacteria use this type of motion, sometimes called “twitching motility,” quite often. Describe the components of a locomotion system that might allow a bacterium to glide along a surface.
Suggested Reading
Wall D, and Kaiser D [1999]. Type IV pili and cell motility. Mol. Microbiol., 32: 1–10. Wolgemuth C, et al. [2002]. How myxobacteria glide. Curr. Biol., 12: 369–377.
