
Molecular and Cellular Signaling - Martin Beckerman
.pdf
17.9 Flagellar Motors Are Erected in Several Stages |
421 |
FIGURE 17.4. Crystal structure of the Lac repressor bound to a DNA operator determined using X-ray crystallography: Several domains of the Lac repressor are involved in the interactions. The headpiece domain binds to the DNA in the major groove while the Hinge helix binds DNA in the minor groove. The core domain is shown broken down into NH2 and CO2 subdomains that mediate interactions with inducers and repressors. One other domain, the tetramerization domain that mediates interactions with other LacI proteins, is not included in the figure, but can be seen in other crystal structures. The figure was generated using Protein Explorer with atomic coordinates deposited in the Protein Data Bank under accession code 1EFA.
Hinge helix domains, bind to the DNA in its major and minor grooves, respectively. The core domain enables the repressor to be regulated by allolactose, which induces allosteric transitions that prevent the protein from binding DNA.
Regulatory proteins such as CAP are able to bend DNA. This ability can be seen in Figure 17.5 where a CAP dimer is shown bound to its regulatory DNA regions. The bending of the DNA is quite pronounced, and by this means transcription factors and coactivators can make transcription either easier or harder.
17.9 Flagellar Motors Are Erected in Several Stages
Bacteria can alter their morphology, either building up or disassembling internal structures, such as gas vesicles, and membrane wall-attached structures, such as flagellar motors, pili, and virulence cassettes. It takes more than 50 genes to assemble a flagellar motor system. In species such as

422 17. Cell Regulation in Bacteria
FIGURE 17.5. Crystal structure of DNA bending by a CAP dimer determined using X-ray crystallography: The figure was generated using Protein Explorer with atomic coordinates deposited in the Protein Data Bank under accession code 1RUN.
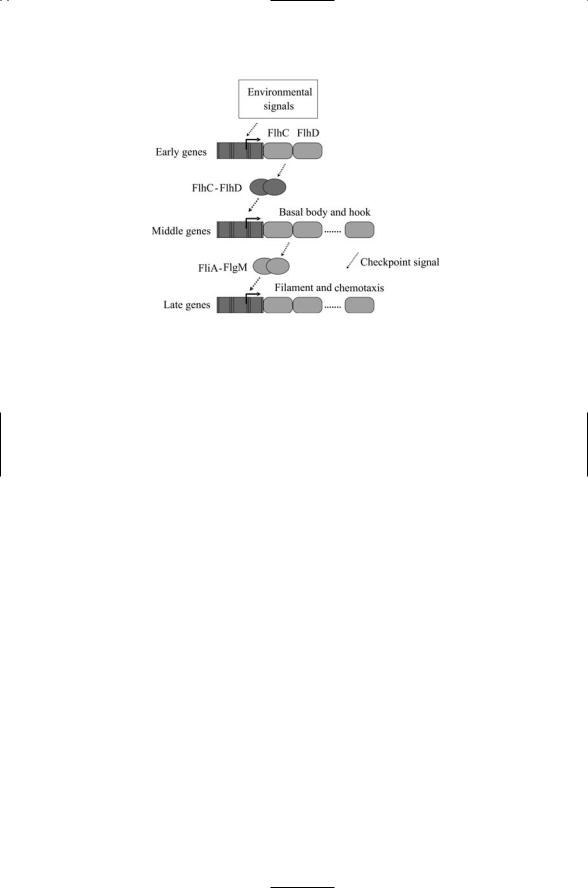
Escherichia coli and Salmonella enterica, these genes belong to a large flagellar motor regulon. The genes in this regulon are organized temporally into three classes—early, middle, and late (Figure 17.6).
A variety of cellular and environmental conditions are factored into the decision to begin biosysnthesis of the flagellar motor. The corresponding signals impinge on a master control point, namely the promoter for the flhCD master operon, from which the early genes flhC and flhD are transcribed. The transcriptional activation complex then regulates the transcription of middle genes. These genes encode structural proteins and assembly regulators for the basal body and hook of the flagella. The timing of events is carefully controlled during the assembly process. Two regulatory proteins are expressed during the middle phase. One of these, FlgM, binds to and inhibits the second, FliA (s28). The inhibition on s28 by FlgM is relieved after the middle stage components of the flagellar motor have been assembled. The s28 regulator then initiates transcription of the late genes including those for the chemotactic receptors Tar, Tsr and Aer, and the suite of Che proteins discussed in Chapter 6.
17.10Under Starvation Conditions, B. subtilis Undergoes Sporulation
Under starvation conditions, B. subtilis undergoes a program of asymmetric cell division resulting in two daughters, a large “mother cell” and a small prespore that further develops into a spore that, in turn, aids in long term survival. When nutrients are plentiful B. subtilis enters a vegetative state where it multiplies exponentially. The vegetative growth sigma factor sA governs this state. Under starvation conditions, B. subtilis alters its meta-
17.10 Under Starvation Conditions, B. subtilis Undergoes Sporulation |
423 |
FIGURE 17.6. Assembly of the flagella motor: Environmental signals arriving at the master controller stimulate transcription of early (Class I) genes. FlhC and FlhD jointly stimulate transcription of middle genes from middle (Class II) promoters. FliA (s28) and FlgM (anti-s28) regulate transcription from late (Class III) promoters. Transcription of late genes is inhibited until completion of the basal body and hook assembly signified in the figure by the sending of a checkpoint signal.
bolic and reproductive strategy. It leaves the vegetative state and converts itself into an inactive spore that can survive for long periods of time. As indicated in Table 17.3 five sigma factors govern the transition from vegetative growth to spore. The first to come into play is sH. This sigma factor is followed in sequential fashion by sF, sE, sG, and finally sK. As the genes selected by these sigma factors are expressed the bacterium ceases exponential growth. Its plasma membrane invaginates, partitioning the cell into large and small compartments, each containing a chromosome generated during the last round of DNA replication. Once the septum is completed the large compartment, the mother cell, engulfs the small cell, the forespore, producing a cell within a cell. A series of coating stages follow where the forespore and its chromosome are protected, and lastly the spore is released through lysis of the mother cell; that is, the rupture of the plasma membrane/cell wall and the dissolution of the outer cell.
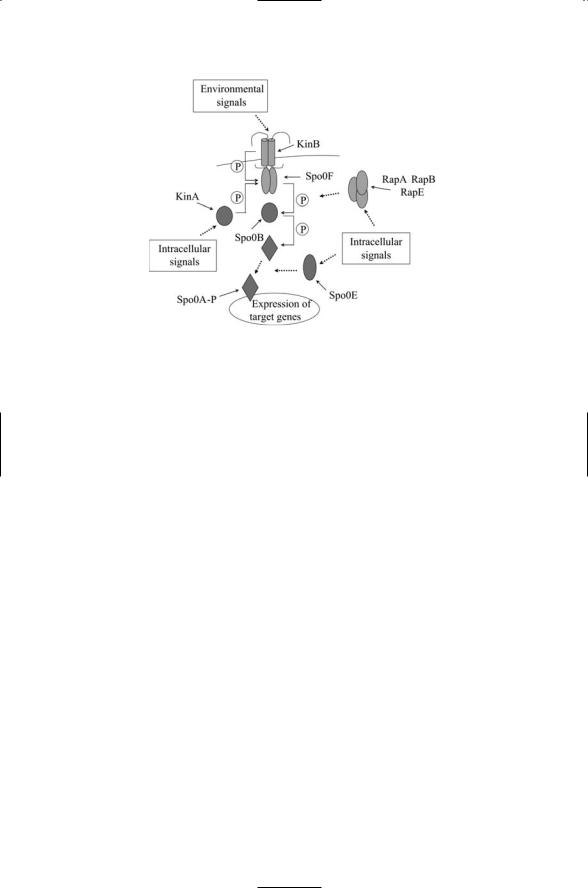
A number of signals, integrated together, govern the decision whether to undergo sporulation (Figure 17.7). These signals converge on the transcription factor Spo0A. This protein acts at the end of a phosphorelay system involving kinases such as KinA/KinB, a phosphorelay protein Spo0B and an upstream transmitter Spo0F. Signals sent through the phosphorelay, and associated protein phosphatases such as RapA, RapB, RapE, and Spo0E include metabolic information, DNA status, and cell density. These
424 17. Cell Regulation in Bacteria
FIGURE 17.7. Integration of sporulation signals: Environmental and intracellular signals are integrated together into a phosphorelay consisting of at least two sensor (input) kinases (KinA and KinB) a Spo0F transmitter, Spo0B intermediate, and Spo0A response regulator, which functions as a transcription factor when phosphorylated. Negative regulatory signals are conveyed by several aspartyl phos- phatases—Rap family members dephosphorylate Spo0F and Spo0E dephosphorylates Spo0A.
signals influence the phosphorylation status of Spo0A. The Spo0A transcription factor, along with KinA and Spo0F, is upregulated by sH. The Spo0A operon contains the genes for sF and sE, and starts the expression of spore and mother-specific genes.
17.11Cell-Cycle Progression and Differentiation in
C. crescentus
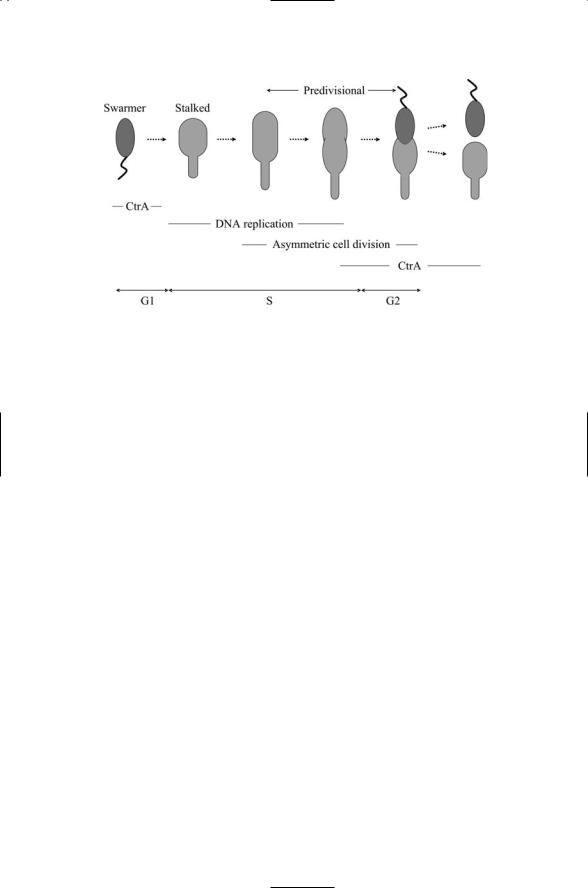
C. crescentus is a gram-negative bacterium adept at survival in a variety of aquatic environments. During every cell cycle it differentiates, producing as one offspring a motile swarmer cell equipped with a single flagellum at one pole, and as the second offspring an immobile (sessile) stalked cell possessing a stalk rather than a flagellum. While stalked cells are immediately competent to undergo cell division, the swarmer is not. It must first convert itself into a stalked cell. The swarmer possesses a single flagellum at one pole, and this flagellum is coupled to a chemotactic sensor system. During G1 phase, the swarmer can move about seeking nutrients using its chemotactic system. At the beginning of S phase (Figure 17.8), the swarmer degrades the flagellum and chemotactic sensor system. A stalk develops at the pole where the flagellum was sited, and the cell elongates. The DNA is
17.11 Cell-Cycle Progression and Differentiation in C. crescentus |
425 |
FIGURE 17.8. Asymmetric cell division in Caulobacter crescentus: A swarmer cell differentiates into a stalked cell, which then undergoes asymmetric cell division to produce a swarmer cell and a stalked cell. CtrA gene expression suppresses DNA replication. This master controller is shut down through regulated proteolysis at the end of G1 phase and is started up again in the new swarmer cell near the end of S phase.
replicated, the chromosomes move to opposite ends of the cell, and a new flagellum is formed at the other pole. In the next phase, G2, the cell divides into a swarmer cell and a stalked cell.
The key element in the regulatory network that coordinates the morphological changes with the cell cycle progression is CtrA. This regulatory protein is the counterpart to the Spo0A transcription factor discussed in the last section. The CtrA master cell cycle controller controls a large number of genes, in this case, about 25% of all the genes whose expression varies in a cell cycle-dependent way in C. cresentus. Cellular localization, proteolysis, and phosphorylation all have role in regulating the behavior of CtrA. The controller is expressed in the swarmer, but not in the stalker. The CtrA proteins that are present in the swarmer at the end of G1 phase undergo proteolytic destruction and thus are absent in the S phase starker. Pole proteins belonging to the flagellum and chemotactic sensor are also cleared by proteolysis. These and the subsequent regulatory processes are facilitated by a number of two-component signal transduction systems that localize to one or the other of the poles. One of these systems is DivJ/DivK. This two-component system facilitates the localization of CtrA to the pole that will produce a new flagellum and upon cell division will belong to the new swarmer. Another protein, the histidine kinase CckA phosphorylates CtrA after DNA replication takes place, thereby activating CtrA’s transcriptional activities. In response, CtrA regulates the transcription of genes involved in flagellar biogenesis, cell division, DNA methylation, and DNA

426 17. Cell Regulation in Bacteria
replication. In this way, localization, proteolysis, and phosphorylation pattern the spatial and temporal actions of CtrA, enabling it to coordinate morphogenic and cell cycle activities.
17.12Antigenic Variation Counters Adaptive Immune Responses
Bacteria can upand downregulate expression of their genes fairly rapidly and can move DNA about. One of the ways bacteria (and a number of singlecelled eukaryotes) exploit such capabilities is through antigenic variation, the systematic alterations in antigens expressed on the cell surface.Antigenic variation is used by a number of vector-borne bacterial and protozoal pathogens to defeat the acquired immune response of vertebrates. The vectors are typically arthropods—tics, lice, flies, and mosquitoes.Well-studied pathogens include several species of Borrelia responsible for relapsing fever, African Trypanosomas, the protozoan causative agent of sleeping sickness, and Plasmodium falciparum, the protozoan responsible for malaria.
As discussed in Chapter 9, the acquired (adaptive) immune response takes several days to develop. It requires several steps: First there is antigen recognition, then communication and convergence of leukocytes to the site of infection, and finally clearance. In antigenic variation, the pathogen utilizes the differential expression and switching between members of a family of antigens among a population of clones. Multiple copies of the gene encoding the antigen, each differing somewhat in its recognition epitope, are maintained by the pathogen. By either differentially expressing different copies among different clones, or by switching from one copy to another more rapidly than the response time of the immune system, the pathogens evade recognition and simply wear out the immune system.
A variety of switching mechanisms are employed. Most are implemented at the transcription level. One method is to silence a promoter at one site and turn on expression at another. Alternatively, point mutations may be used, or a gene from a silent site may replace another at an active site of transcription through gene rearrangements. Still another method, one that does not operate at the transcription level, is to differentially transport the antigens to the surface. No matter what the mechanism, the result is the same. The population of pathogens survives.
17.13Bacteria Organize into Communities When Nutrient Conditions Are Favorable
Bacteria talk to each other and share genetic material. Bacteria continually secrete communication molecules that are sensed by other bacteria. The signaling informs the bacteria that others are present in their vicinity and

17.13 Bacteria Organize into Communities |
427 |
TABLE 17.6. Bacteria and their collective behavior.
Bacterial species |
Collective behavior |
Gram-negative bacteria |
|
Photobacterium fischeri |
Luminescence |
Vibrio harveyi |
Luminescence |
Proteus mirabilis |
Swarming, migratory rafts |
Serratia liquefaciens |
Swarming, migratory rafts |
Myxococcus xanthus |
Fruiting body |
Chondromyces crocatus |
Fruiting body |
Pseudomonas aeruginosa |
Antibiotic resistance, virulence factors |
Escherichia coli |
Virulence factors |
Gram-positive bacteria |
|
Staphylococcus aureus |
Virulence factors |
Streptomyces coelicolor |
Antibiotics, differentiation |
Bacillus subtilis |
Antibiotics, genetic competence |
Streptococcus pneumoniae |
Genetic competence |
|
|
enables them to sense their numbers. If the density of bacteria exceeds a threshold for cooperative behavior then the bacteria express genes that support that form of behavior. Any of a number of collective behaviors may be exhibited, each depending on the species, its needs, and its environmental conditions. A sapling of these collective behaviors is presented in Table 17.6.
When nutrient conditions are favorable so that migration is not necessary bacteria organize into highly structured communities. In forming these associations the bacteria express sets of genes that differ from those utilized when free-living. The members of the bacterial communities differ in morphology and physiology from their planktonic counterparts. In communities that are anchored to solid surfaces, the bacteria can develop elaborate three-dimensional structures. These colonies take on characteristics usually associated with multicellular organisms. They exhibit simple forms of differentiation and cellular specialization. The bacteria excrete molecules that form a matrix, form channels that support the circulation of fluids, and share metabolic tasks. Some bacterial colonies are conical-shaped while others are mushroom-shaped and grow to sizes that allow the naked eye to observe them.
The first bacteria discovered to form communities were the luminescent marine organisms Photobacterium fischeri and Vibrio harveyi. These bacteria were found to form symbiotic associations with several species of marine fish and squids. The luminescent bacteria collect in the light organs of their marine hosts. The concentrations of the bacteria are generally low in seawater, typically on the order of 100 cells per milliliter, well below any threshold for luminescent behavior. However, the bacteria can reach high concentrations, 1010 to 1011 cells/ml under favorable nutrient conditions in the light organ. Once the threshold density is exceeded the bacteria collec-

428 17. Cell Regulation in Bacteria
tively express genes for luminencence. The relationship between bacteria and animal host is a symbiotic one. The production of light by the bacteria masks the host from prey residing below them by reducing the host’s shadow against the sky, while the hosts provide nutrients for the bacteria.
Table 17.6 lists several prominent forms of communal behavior. One of these is the production of antibiotics. These are natural byproducts of cellular metabolism. They are agents produced by microorganisms (fungi and bacteria) that kill or inhibit other microorganisms. Fungi produce penicillin and a closely related family of antibiotics, the cephalosporins. Streptomyces produce tetracycline, streptomycin, and erythromycin. These natural products interfere with protein synthesis and damage cellular membranes. Bacillus subtilis produces bacitracin, an antibiotic often added to animal feed.
Other widely utilized forms of coordinated behavior are the production of virulence factors and genetic competence. Virulence factors (e.g., toxins) are cellular products that enhance bacterial survival in hostile host environments. Natural genetic competence is the ability of bacteria to acquire large pieces of exogenous (extracellular) DNA from their local microenvironment.
17.14Quorum Sensing Plays a Key Role in Establishing a Colony
Bacteria use cell-to-cell signaling to take a census of how many fellow bacteria are present in their local environment. The cell density-determining process is known as quorum sensing, and it operates in the following manner. The bacteria secrete signaling molecules called autoinducers. When the local environment is restricted in some manner so that the molecules do not simply diffuse away, the concentration of autoinducers will increase in a manner that reflects the number of bacteria secreting the molecules. The quorum sensing system responsible for secreting autoinducers and sensing their concentration is an integral part of the bacterial cell control system. Once the concentration exceeds a threshold level, a program of gene expression is initiated that leads to alterations in morphology and physiology, and supports communal behavior.
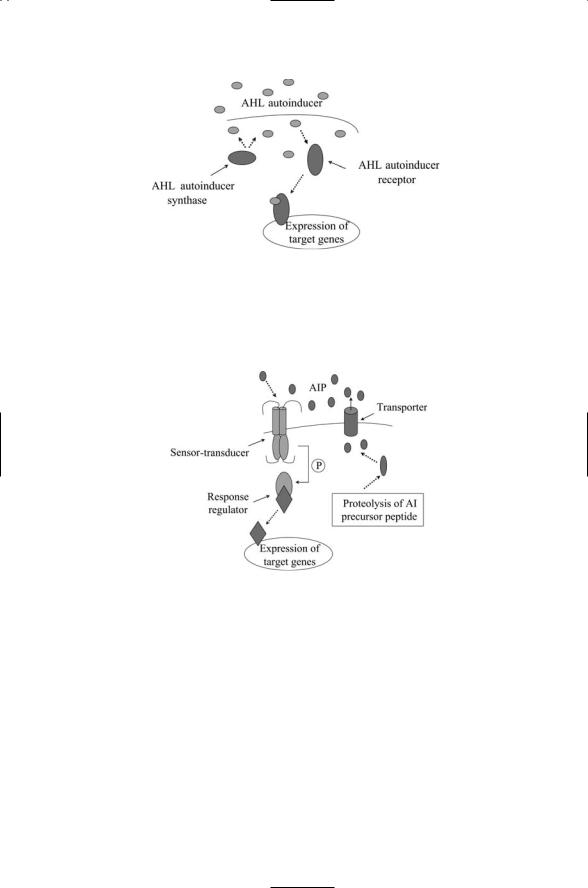
Many different kinds of signaling molecules are utilized in cell-to-cell communication. Some of these are species-specific while others enable the bacteria to communicate between members of different species. Gram-neg- ative bacteria utilize short chain acetyl homoserine lactases (AHLs) to signal one another (Figure 17.9). These molecules are able to diffuse across the cell membranes and bind to cytoplasmic receptors of the recipient bacterium. Gram-positive bacteria utilize oligopeptide autoinducers (AIPs). Signaling by oligopeptides in gram-positive bacteria resembles signaling by peptide hormones in eukaryotes. The autoinducers do not diffuse across the
17.14 Quorum Sensing Plays a Key Role in Establishing a Colony |
429 |
FIGURE 17.9. Quorum sensing in Gram-negative bacteria: One component of the system, the autoinducer synthase generates AHL signaling molecules that freely leave and enter bacterial cells. These are bound by autoinducer receptors, which can then stimulate transcription.
FIGURE 17.10. Quorum sensing in gram-positive bacteria: Signaling peptides are shipped out of the cell by means of a transporter. The signals (autoinducer peptides or AIPs) bind to bacterial two-component signals transduction systems, which transduce signals into the cell and stimulate gene transcription.
membranes. Instead, they are shipped out of the sender by ABC transporters, and diffuse over to and subsequently bind transmembrane receptors of the recipient. The latter receptors operate as the sensor components of two-component signal transduction systems (Figure 17.10).
In some species (e.g., B. subtilis and S. pneumoniae) quorum sensing triggers a bacterial state called genetic competence that is geared for the uptake of exogenous DNA. In others a threshold-exceeding density of cells induces

430 17. Cell Regulation in Bacteria
a virulence response (S. aureus) and the expression of virulence genes (P. aeruginosa), or the formation of a biofilm, a colony of bacteria collectively exhibiting an increased resistance to antibacterial agents.
17.15Bacteria Form Associations with Other Bacteria on Exposed Surfaces
Whenever possible, bacteria establish surface-attached communities known as biofilms. These structures can be formed by members of one species or by members of multiple species. Bacteria can freely switch between freeliving and communal forms of organization, and communities can form, grow, collectively migrate, and disperse. When bacteria organize themselves into biofilms they become difficult to eradicate. The biofilms contain not only the bacterial cells but also a protective matrix of secreted molecules, secreted by the bacteria, that protects the members of the colony from harmful agents in their environment. Actinomyces, Lactobacillus, and Streptococcus films can appear as plaque on teeth; Staphylococcus and Escherichia coli films can emerge on medical instruments and surgical implants, and Pseudomonas aeruginosa and Escherichia coli colonies can form in the body.
Pseudomonas aeruginosa forms elaborate structures on surfaces. This highly adaptive species is an opportunistic pathogen, and is often encountered in the treatment of cystic fibrosis patients and burn victims. The formation of a biofilm protects the P. aeruginosa from antibiotic applications, increasing their resistance to the antibiotics by more than a hundredfold. These bacteria are highly resistant to antibiotic treatment due in part to the presence of their self-generated polymeric matrix that encases the colony and protects it from the actions of antibiotics.
Biofilms are also encountered in urinary tract infections. Most urinary tract infections are due to uropathogenic strains of Escherichia coli (UPEC). These bacteria are able to form podlike bulges on the surface of the bladder. Like the P. aeruginosa biofilms formed in lungs, the UPEC cells are encased in a protective shell that insulates them from antibiotics. They are able to persist as pods for several months undetected, and are utterly resistant to standard courses of antibiotics.
17.16 Horizontal Gene Transfer (HGT)
Bacterin share genetic information through horizontal gene transfer
(HGT). Horizontal gene transfer differs from vertical gene transfer that takes place between parents and their offspring. In HGT genetic information is transferred between distantly related species. There are several different mechanisms for affecting the lateral transfer of genetic material. One
