
Molecular and Cellular Signaling - Martin Beckerman
.pdf
512 21. Learning and Memory
movements. It allows associations to be made that are needed for survival (for example, of sights, sounds, touches and odors indicative of danger), and makes possible learning and remembering and forgetting.
The preand postsynaptic terminals of nerve cells are highly enriched in signaling molecules. Most major classes of signaling molecules discussed in the preceding chapters are present in the synapses. Thee include ion channels, enzymes such as protein kinases, protein phosphatases and GTPases, G protein-coupled receptors, cell adhesion molecules, and nonenzymatic scaffold, adapter, and anchor proteins. The molecular composition of the synapses will be examined following a brief overview of how neurons are organized in the brain. The remainder of the chapter is devoted to explorations of learning and memory formation. The preeminent experimental model of how this is accomplished is called long-term potentiation. It is studied most often in vitro using slice preparations from the hippocampus of rodents. Findings from these experiments as well as from experiments of fear conditioning and drug dependence, which can be thought of as an aberrant form of learning and memory, will be explored, as well.
21.1 Architecture of Brain Neurons by Function
Neurons in the brain are organized into architecturally distinct functional areas. The large-scale division of the brain into brain stem, limbic system, cerebellum, and cerebral cortex is depicted in Figure 21.1. The first of the aforementioned large-scale regions, the brain stem, contains a number of structures whose activities were discussed in the previous chapter. Neurons in the medulla oblongata and pons regulate rhythmic movements associated with breathing and heartbeat, while cells in the reticular activating complex control alertness and the sleep/wake cycle. The limbic system is situated just above the brain stem. It includes the hippocampus, and the amygdala, which mediates emotional responses such as fear and aggression.
FIGURE 21.1. Architecture of the brain showing its major divisions: Shown is a side view with the front of the head on the left and the back of the head on the right. The partitioning of the cerebral cortex is depicted along with the locations of the brain stem, cerebellum, and limbic system.

21.1 Architecture of Brain Neurons by Function |
513 |
FIGURE 21.2. Architecture of the brain showing the sequestering of brain functions within specific areas of the brain: The locations of the major sensory areas are shown along with motor areas and sites where forms of learning and memory, and higher processing functions, as most strongly associated.
The cerebellum is a large region that sits behind the brain stem and below the occipital lobe. Neurons in this region of the brain integrate and coordinate motor actions and mediate motor learning. Cerebellar neurons control balance and posture, and ensure that fine motor actions are accurate. The large section of cortex sitting above and about these structures is partitioned into frontal, parietal, occipital, and temporal lobes. The temporal lobe sits above and to the outside of the brain stem at about the level of the ears. As indicated in Figure 21.2, neurons in this region are involved in the senses of sound,taste,and smell,and in memories thereof. Information of a tactile character, that is, the sense of touch, is processed by neurons located in the somatosensory cortex that lies above in the parietal lobe. This region is situated next to regions where associations and interpretations are made from information sent from the primary visual cortex and other primary sensor cortices. Behind this region,at the back of the head in the occipital lobe,(primary) visual information is received and processed. Lastly, the nonmotor portions of the frontal cortex are used for making judgments and intelligent decisions. The premotor cortex controls head and eye movements, while speech is centered in Broca’s area and smell in the olfactory center.
The presence of a large region devoted to motor and sensory information processing is unique to mammals. This region is highly differentiated radially into layers and laterally into regions. As first noted by Brodmann in 1909, regions of cortex involved in processing sensory information are organized in the radial direction into six layers and in the lateral direction into at least 50 distinct areas. The numbers of sublayers, and their thicknesses and arrangements, vary from area to area in the cortex. Within each area there are cells of a particular size and arborization, with a distinct set of inputs, outputs, and

514 21. Learning and Memory
intercortical connections. The overall organization of the neocortex is that of a patchwork of areas each with its own cellular organization, or cytoarchitecture. Brodmann based his partitioning of the cortex upon differences in appearance that he was able to observe using a light microscope. In more modern approaches, functional MRI and other biophysical methods are used to assign functions to anatomical regions. The anatomical regions, called Brodmann’s areas,correspond approximately to functional areas,so that each anatomically distinct area carries out some unique function.
21.2 Protein Complexes’ Structural and Signaling
Bridges Across Synaptic Cleft
Communication occurs when neurotransmitters released from the presynaptic terminal diffuse across the synaptic cleft and bind to receptors on the postsynaptic side. Before this communication can occur, a minimal set of molecular components must be assembled into a functional unit, the synapse. Active zones on the presynaptic side that contain the machinery for neurotransmitter release must align with the postsynaptic density (PSD) on the postsynaptic side containing the receptors for the neurotransmitters. These activities must take place as the two parts of the signaling unit are formed.
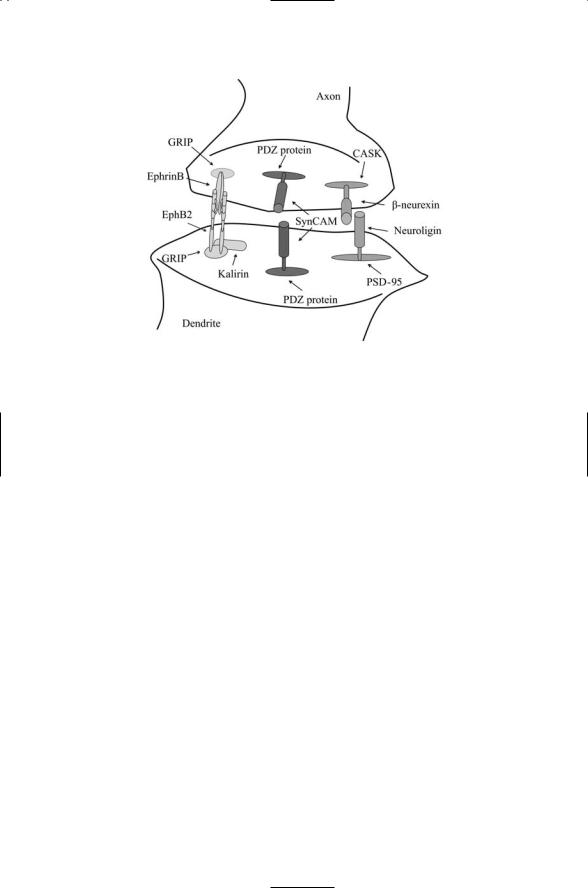
These preand postsynaptic portions of the synapse are aligned and stabilized by proteins that form bridges from one side of the cleft to the other. Several of the most prominent bridging pairs—Neurexins-to-Neuroligins, SynCAMs-to-SynCAMs and EphB2s-to-EphrinBs—are depicted in Figure 21.3. Neurexins are brain-specific cell surface proteins containing one or more LNS (laminin, neurexin, sex hormone-binding globulin) domains. The LNS domains, approximately 190 amino acid residues in length, act as cell surface recognition modules. They are found in a variety of extracellular matrix proteins and in cell surface receptors, typically in combination with multiple EGF repeats. Neuroligins act as ligands for Neurexins. They are brain-specific, single-pass, transmembrane proteins found on the opposing surface to the neurexins. Neurexin-Neuroligin cell-cell adhesion complexes form a bridge between the preand postsynaptic terminals. Neurexin interacts with CASK on the presynaptic side, and neuroligin binds to the PSD95 proteins on the postsynaptic side. The interactions with each another and with the cytoplasmic CASK and PSD-95 proteins stabilize and maintain the alignment between active zones on the presynaptic side and the postsynaptic density at the postsynaptic terminal.
More than one kind of signaling complex ties together the active zone on the presynaptic side and the PSD at the postsynaptic terminal. Members of the immunoglobulin superfamity of cell adhesion molecules called “synCAMs” establish homophilic contacts between proteins expressed on the two opposing surfaces. As is the case for other proteins that perform a structural role mediating surface adhesion, the synCAMs also have a
21.3 The Presynaptic Terminal and the Secretion of Signaling Molecules |
515 |
FIGURE 21.3. Transsynaptic protein–protein interactions: Protein pairs form bridges that physically link preand postsynaptic terminals. The protein pairs are tied to scaffolding proteins on the two sides of the synapse. Many, if not most, of the scaffolding proteins have PDZ domains. Kalirin, which binds EphB2, is a Rho GEF.
signaling role. They appear to supply signals telling nascent presynaptic terminals to differentiate into a mature signaling form. The neurexinneuroligin complexes serve a similar signaling role. These too instruct the cells to form differentiated presynaptic terminals. Several families of cell adhesion molecules participate in bridging actions. Neuron-specific cadherins form bridging complexes, too. These proteins are anchored at both sides of the synaptic cleft to b-catenins.
Another family of proteins having an important role in synapse formation is the EphB receptor tyrosine kinases and their Ephrin ligands. The EphB2 receptor signals through the Rho GEF kirilin to the actin cytoskeleton, thus helping in dendritic spine morphogenesis. In synapses that signal using glutamate, N-methyl-D-aspartate (NMDA) receptors are an early occupant of the postsynaptic density. Kirilin helps establish NMDA receptor aggregates at newly created synapses.
21.3 The Presynaptic Terminal and the Secretion of
Signaling Molecules
When action potentials arrive at the presynaptic terminal, voltage-gated calcium channels open and calcium ions flow into the terminal. The calcium ions bind to calcium sensors attached to the neurotransmitter-filled vesicles.

516 21. Learning and Memory
FIGURE 21.4. Neurotransmitter release at the presynaptic terminal: In response to the arrival of an action potential, calcium enters the cell through voltage-gated Ca2+ channels (VGCCs) and triggers fusion and release of neurotransmitters from neu- rotransmitter-filled synaptic vesicles. The neurotransmitter molecules diffuse across the synaptic cleft and bind to receptors embedded in the terminal membrane of the postsynaptic cell.
Binding of the calcium to the sensor triggers conformational changes leading to vesicle fusion and release of the contents. Once released, the neurotransmitters diffuse across the synaptic cleft and bind to receptors embedded in the postsynaptic membrane. This set of steps is depicted schematically in Figure 21.4.
The amount of neurotransmitter released at a presynaptic terminal depends on several factors. Synapses in the central nervous systems are, in general, small. Their amount of neurotransmitter released at one time is not only small but also can be quite variable. The amount of neurotransmitter released can,for example,depend on timing. If a large number of pulses arrive at the presynaptic terminal in a short period of time,the pool of available neurotransmitter vesicles can be depleted. The release of neurotransmitter will therefore monotonically decline with each later-arriving action potential. Synapses with large pools will release more neurotransmitter than those with small pools. The term “active zone” refers to the exact site along the presynaptic terminal where the vesicles fuse and release neurotransmitter. The amount of neurotransmitter release will depend on the number of active sites per terminal and on the number and size of the vesicles.
Several protein complexes regulate vesicle movements and neurotransmitter release in the presynaptic terminal. These have been organized under

21.3 The Presynaptic Terminal and the Secretion of Signaling Molecules |
517 |
three headings in Table 21.1. The first set of signaling elements in the table is the voltage-gated calcium channel grouping that converts membrane depolarization into a calcium signal. Included under this heading are a number of signaling proteins that allow neuromodulators to influence the strength of the calcium signals and thus the amount of neurotransmitter released into the cleft.
The second grouping contains vesicle regulators such as the soluble NSF attachment protein receptor (SNARE) complex. SNARE proteins regulate the final stages of neurotransmitter release—docking and fusion of the vesicle with the plasma membrane resulting in release of the vesicle contents into the synaptic cleft. There are two classes of SNARE proteins— those that bind to the vesicle surface (v-SNAREs) and those that attach to the plasma membrane (t-SNAREs). Interactions between the two kinds of SNARES regulate the release of neurotransmitter. One of the v-SNAREs, synaptotagmin, contains a calcium-binding C2 domain and functions as the calcium sensor. Conformational changes brought on by calcium binding are conveyed to the t-SNAREs with which it interacts promoting fusion and vesicle content release.
The other signaling complexes in the vesicle regulator grouping help shepherd vesicles into the presynaptic terminal and to the plasma membrane, and then regulate membrane fusion. Neurotransmitter-filled vesicles
TABLE 21.1. Main components of the presynaptic terminal at CNS synapses: Abbreviations—N-ethylmaleimide-sensitive fusion protein (NSF); soluble NSFattachment protein (SNAP); soluble NSF attachment protein receptor (SNARE); vesicle-associated membrane protein (VAMP); CaMK/SH3/guanylate kinase domain protein (CASK).
Presynaptic terminal component Function
Channels and their modulators
Voltage-gated calcium channels N- and P/Q-type calcium channels; enable calcium to
|
enter the cell in response to membrane depolarization |
GPCRs |
Gbg subunits negatively regulate N-type channels |
Protein kinase C |
Positively regulates N-type channels |
Vesicle regulators |
|
v-SNAREs |
Synaptobrevin (VAMP) and synaptotagmin mediate |
|
vesicle docking and fusion. Synaptotagmin functions |
|
as a calcium sensor |
t-SNAREs |
Syntaxin and SNAP-25 mediate vesicle docking and |
|
fusion |
Sec6/8 complex |
Targeting of vesicles to the plasma membrane |
Sec1/Munc18 |
Regulators of Syntaxin |
NSF, a-SNAP |
Dissociation of the SNARE complex |
Scaffolds and adapters |
|
CASK |
Attaches to N- and P/Q-type channels and Mint1; aligns |
|
vesicles |
Mint1 |
Attaches to CASK and N- and P/Q-type channels |
Veli |
Member of the CASK, Mint1, Veli adapter complex |
|
|

518 21. Learning and Memory
are transported along the cytoskeleton into the presynaptic terminal. Upon arrival at the terminal the vesicles are targeted to the plasma membrane by a multiple subunit protein complex called Sec6/8. Another set of proteins, Sec1/Munc18, act both as positive and as negative regulators of SNAREmediated fusion through interactions with Syntaxin. Yet another set of molecules, NSF and a-SNAP, direct the dissociation of the SNAREs permitting their reuse.
The third grouping presented in Table 21.1 encompasses the scaffold and anchor proteins. These proteins help organize the complexes in the presynaptic terminal. As is the case in any cell, these proteins provide attachment sites where proteins that must work together can bind in close proximity to one another.
21.4 PSD Region Is Highly Enriched in
Signaling Molecules
The region just below the postsynaptic membrane is the postsynaptic density (PSD). The PSD is a disclike structure that sits opposite the active zone in the presynaptic terminal (Figure 21.5). It is highly enriched in struc-
FIGURE 21.5. The postsynaptic density: Shown as some of the types of signaling proteins that are found in the postsynaptic density at excitatory synapses in the central nervous system. Glutamate released at the presynaptic terminal diffuses across the synaptic cleft and binds to NMDA and AMPA receptors leading to membrane depolarization, calcium influx, alterations in AMPA receptor population, and gene expression. Short-term modulatory effects are mediated by GPCRs such as mGlu5R, leading to G protein subunitand second messenger-binding to ion channels, and phosphorylation of ion channels by protein kinases.

21.4 PSD Region Is Highly Enriched in Signaling Molecules |
519 |
TABLE 21.2. Main components of the PSD at excitatory glutamergic synapses: Abbreviations—a-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA); N- methyl-D-aspartate (NMDA); calcium/calmodulin-dependent protein kinases II and IV (CaMKII and CaMKIV); glutamate receptor interacting protein (GRIP); guanylate kinase-associated protein (GKAP).
PSD component |
Function |
Receptors |
|
AMPA receptor |
GluRs: Key component of active synapses |
NMDA receptor |
NRs: Dual voltage and ligand-gated allowing entry of |
|
calcium into the cell |
Metabotropic receptors |
mGluRs: Signal to IP3 regulated intracellular calcium |
|
stores |
Kinases and phosphatases |
|
aCaMKII and CaMKIV |
Central signaling element, involved in both short and |
|
long term forms of synaptic plasticity |
Protein kinase A |
Central signaling element, involved in both short and |
|
long term forms of synaptic plasticity |
Protein kinase C b, g isoforms |
Signaling element, involved in short term forms of |
|
synaptic plasticity |
ERK2 type MAPKs |
Signaling element, involved in both short and long term |
|
forms of synaptic plasticity |
Protein phosphatase 1 |
Regulator of homeostasis and learning |
Anchors, scaffolds, and adapters |
|
AKAP79 |
Sequesters protein kinase A, protein kinase C, and |
|
calcineurin near receptors and ion channels |
GRIP |
Attaches to GluR2 and GluR3 |
PSD-95 |
Attaches to NR2s |
SynGAP |
Activates MAP kinase signaling |
GKAP |
Link between PSD-95 and GKAP |
Shank |
Link between Homer and GKAP |
Homer |
Attaches to mGluRs and to Shank |
|
|
tural (cytoskeleton) and regulatory (receptor, ion channel, scaffolding, signaling) proteins. The several different kinds of regulatory proteins have been placed into three groupings in Table 21.2. The first group contains the receptors for neurotransmitters. Glutamate is the predominant neurotransmitter at excitatory synapses in the central nervous system. Several different kinds of glutamergic receptors are found in the PSD of excitatory synapses in the CNS. Some glutamergic receptors are ion channels (ionotropic) while others are G protein coupled receptors (metabotropic). Two kinds of ionotropic glutamate receptors—the a-amino-3-hydroxy-5- methyl-4-isoxazolepropionate (AMPA) receptors and the N-methyl-D- aspartate (NMDA) receptors—are intimately connected with synaptic plasticity. Rather than listing a large number of receptors in the table, only the three main classes of glutamergic receptors have been included in the table. These are the principal receptors involved in learning and memory formation.

520 21. Learning and Memory
The second group in the table consists of several different kinds of serine/ threonine kinases and phosphatases that are central signaling elements in the postsynaptic density. The third grouping contains connecting and supporting agents such as GTPases functioning as molecular adapters, and anchoring and scaffolding proteins that help organize the signaling complexes. These signaling elements form a matrix within the PSD that allows ion channels to cluster together in close proximity to second messenger and downstream signaling elements. The postsynaptic density is enriched in cytoskeleton proteins, especially in actin and actin-related cytoskeleton components.
21.5 The Several Different Forms of Learning
and Memory
There are several different kinds of learning. The forms of learning described earlier with regard to Tritonia and Aplysia—sensitization and habituation—are nonassociative in character. Repeated stimulation of noxious stimuli produce sensitization while repeated exposures to a harmless stimulus lead to habituation, a reduced responsiveness. These forms of learning do not require a second pairing stimulus to elicit behavioral changes.
Associative learning, in contrast, involves a pairing of stimuli. The earliest and still most famous example of associative learning is Pavlov’s dog experiments. In these and similar experiments, an initial nondangerous event, called the conditioned stimulus (CS), is paired repeatedly with a biologically significant (potentially dangerous) event called the unconditioned stimulus (US). Over time, the relationship between the CS and US is learned; that is, the behavioral responses adapt in response to the experiences. In a typical laboratory experiment, a rat is given a tone, a neutral nondangerous CS, which is paired with an electrical shock, the dangerous US. After as few as one or two exposures, the tone will elicit a number of physiological and behavioral adaptations. The rat will freeze in place and exhibit reflex actions. Heart rate and blood pressure will change, and stress hormones will be released. In Aplysia, the associative learning counterpart is called long-term facilitation. In this process, an electric shock to the tail serves as the US, and a weak touch to the siphon functions as the CS. The snail learns to associate the two stimuli and, because of the association with the electrical shock, alters its behavioral response to the weak touch over time.
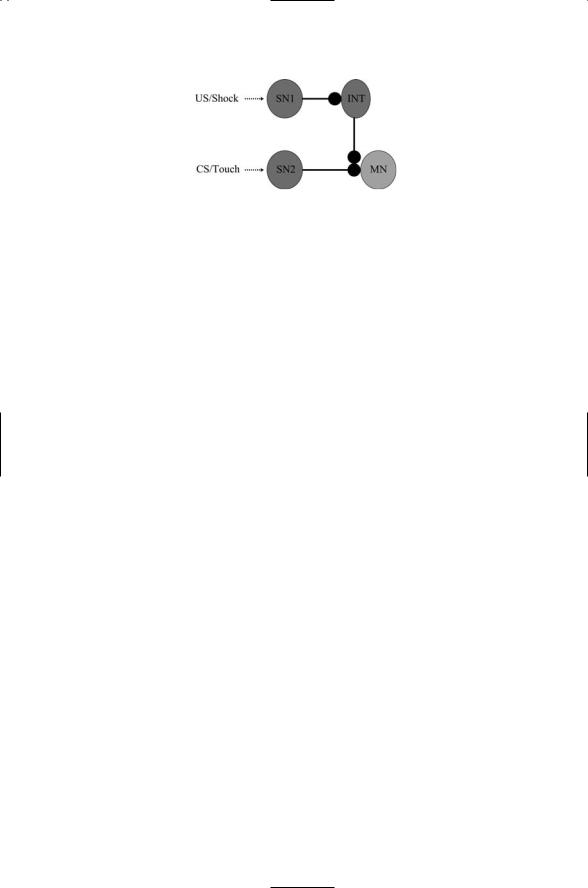
The circuitry responsible for the Aplysia gill withdrawal response is depicted in Figure 21.6. The axon of the sensory neuron that conveys touch signals contacts a dendrite on a motor neuron. The sensory neuron that conveys the electric shock signals also makes contact with the motor neuron. As shown in the figure the neuron has a synaptic connection with an interneuron that releases serotonin from its axon terminal. The serotonin
21.6 Signal Integration in Learning and Memory Formation |
521 |
FIGURE 21.6. Circuitry underlying the associative Aplysia gill withdrawal response: Three different kinds of neurons form the circuit—sensory neurons that detect and respond to electrical shock (SN1) and touch (SN2), a serotonin-releasing interneuron (INT), and a motor neuron (MN).
diffuses across the synaptic cleft to the axon terminal of the touch sensory neuron where the two sets of signals converge.
Just as there are several types of learning, there are several kinds of memory. Short-term memory refers to memories that are labile (responsive to changes in conditions under which they are created) and are only transiently formed. They are lost if not converted into stable memory traces. Short-term labile memories involve celluar changes that utilize cellular resources that are already available and are local to the synapse or synapses undergoing modification. In contrast, long-term stable memory traces typically involve gene expression and protein synthesis leading to architectural modifications that produce the lasting changes in synaptic transmission.
This events stimulated by the release of serotonin are depicted in Figure 21.7. These include stimulation of adenylyl cyclase leading to the production of cAMP and its subsequent activation of the catalytic subunits of protein kinase A. Protein kinase A phosphorylates the nearby potassium channels and, as a result, currents through potassium channels are reduced.
The depolarization accompanying arrival of the action potential in the axon terminal lasts longer. More calcium is able to enter the terminal resulting in a greater release of neurotransmitter. Two routes are illustrated in the figure. One of the routes leads to ion channel modification, a short-term memory trace. The other route leads to the nucleus and results in changes in the pattern of gene expression, producing a longer lasting memory trace.
21.6 Signal Integration in Learning and
Memory Formation
Synapses were first described by Ramón y Cajal in the 1890s. It took several years for the idea to be accepted that nerve cells were not hard-wired to one another, but instead communicated using chemical messengers that diffuse across a narrow gap between preand postsynaptic terminals. The
