
Molecular and Cellular Signaling - Martin Beckerman
.pdf
References 461
Sodeik B [2000]. Mechanisms of viral transport in the cytoplasm. Trends Microbiol., 8: 465–472.
Sodeik B, Ebersold MW, and Helenius A [1997]. Microtubule-mediated transport of incoming herpes simplex 1 capsids to the nucleus. J. Cell Biol., 136: 1007–1021.
Viral Movement In and Out of the Nucleus
Salman H, et al. [2001]. Kinetics and mechanism of DNA uptake into the cell nucleus. Proc. Natl. Acad. Sci. USA, 98: 7247–7252.
Trotman LC, et al. [2001]. Import of adenovirus DNA involves the nuclear pore complex receptor CAN/Nup214 and histone H1. Nature Cell Biol., 3: 1092–1100.
Viral Release and Budding
Freed EO [2002]. Viral late domains. J. Virol., 76: 4679–4687.
Katzmann DJ, Odorizzi G, and Emr SD [2002]. Receptor downregulation and multivesicular-body sorting. Nature Rev. Mol. Cell Biol., 3: 893–905.
McDonald D, et al. [2003]. Recruitment of HIV and its receptors to dendritic cell- T cell junctions. Science, 300: 1295–1297.
Pelchen-Matthews A, Kramer B, and Marsh M [2003]. Infectious HIV-1 assembles in late endosomes in primary macrophages. J. Cell Biol., 162: 443–455.
Von Schwedler UK, et al. [2003]. The protein network of HIV budding. Cell, 114: 701–713.
Hepatitis C Virus
Bartenschlager R, and Lohmann V [2000]. Replication of hepatitis C virus. J. Gen. Virol., 81: 1631–1648.
Goodbourn S, Didcock L, and Randall, RE [2000]. Interferons: Cell signalling, immune modulation, antiviral responses, and viral countermeasures. J. Gen. Virol., 81: 2341–2364.
Katze MG, He YP, and Gale M, Jr. [2002]. Viruses and interferons: A fight for supremacy. Nature Rev. Immunol., 2: 675–687.
Large MK, Kittlesen DJ, and Hahn YS [1999]. Suppression of host immune response by the core protein of Hepatitis C virus: Possible implications for Hepatitis C virus persistence. J. Immunol., 162: 931–938.
HIV-1
Boshoff C, and Weiss R [2002]. Aids-related malignancies. Nature Rev. Cancer, 2: 373–382.
Emerman M, and Malim MH [1998]. HIV-1 regulatory/accessory genes: Keys to unraveling viral and host biology. Science, 280: 1880–1884.
Gu YP, and Sundquist WI [2003]. Good to CU, Nature, 424: 21–22.
Jacotet E, et al. [2000]. Control of mitochondrial membrane permeabilization by adenine nucleotide translocator interacting with HIV-1 viral protein R and Bcl- 2. J. Exp. Med., 193: 509–519.
Karn J [1999]. Tackling Tat. J. Mol. Biol., 293: 235–254.
Kwong, PD, et al. [2002]. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor binding sites. Nature, 420: 678–682.
Lum JJ [2003]. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J. Clin. Invest., 111: 1547–1554.

462 18. Regulation by Viruses
Marin M, et al. [2003]. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nature Med., 9: 1398–1403.
Sheehy AM, Gaddis NC, and Malim MH [2003]. The antiretroviral enzyme APOBEC3G is degraded by the proteosome in response to HIV-1 Vif. Nature Med., 9: 1404–1407.
Stevenson M [2003]. HIV-1 pathogenesis. Nature Med., 9: 853–860.
Swigut T, Shohdy N, and Skowronski J [2001]. Mechanism for downregulation of CD28 by Nef. EMBO J., 20: 1593–1604.
Swingler S, et al. [2003]. Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature, 424: 213–219.
Swingler S, et al. [1999]. HIV-Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nature Med., 5: 997–1003.
Wei XP, et al. [2003]. Antibody neutralization and escapes by HIV-1, Nature, 422: 307–312.
Wyatt R, and Sodroski J [1998]. The HIV-1 envelope glycoproteins: Fusogens, antigens and immunogens. Science, 280: 1884–1888.
KSHV
Damania B, Choi JK, and Jung JU [2000]. Signaling activities of gammaherpesvirus membrane proteins. J. Virol. 74: 1593–1601.
Moore PS, and Chang Y [2001]. Molecular virology of Kaposi’s sarcoma-associated herpesvirus. Phil. Trans. R. Soc. Lond. B, 356: 499–516.
Bacteriophage Lambda
Bell CE, et al. [2000]. Crystal structure of the l repressor C-terminal domain provides a model for cooperative operator binding. Cell, 101: 801–811.
Campbell A [2003]. The future of bacteriophage biology. Nature Rev. Genet., 4: 471–477.
McAdams HH, and Shapiro L [1995]. Circuit simulation of genetic networks. Science, 269: 650–656.
Ptashne M, and Gann A [1997]. Transcriptional activation by recruitment. Nature, 386: 569–577.
Shiga Toxin
O’Loughlin EV, and Robins-Brown RM [2001]. Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect., 3: 493–507.
Wagner PL, and Waldor MK [2002]. Bacteriophage control of bacterial virulence. Infect. Immun., 70: 3985–3993.
Problems
18.1Engineered viruses are being devised for use in cancer therapy. The goal of researchers is to find natural viruses, or create genetically modified ones, that can kill cancer cells while leaving normal cells unharmed. This main idea behind creation of these oncolytic viruses is to take advantage of the altered signaling pathways in the tumors by devising viruses that selectively target those cells.

Problems 463
The first viruses to be used in this way are the adenoviruses. These viruses and other like them stimulate host cells to pass through the cell cycle. If the host cell does not pass into S phase the viruses cannot replicate their DNA. Adenoviruses encode 30 to 40 proteins that are expressed sequentially. First immediate early genes, then early genes and finally late genes are expressed. The first protein to be made is E1A. This control protein binds to the pRB protein of the host cell and inactivates it (Figure 14.11). The E1B protein is made soon thereafter. It binds to p53 and inactivates it, too. These binding and inactivation events ensure entry of the host cells into S phase.
Recall from Chapter 14 that p53 and pRb central cell cycle controller circuitry and are mutated in most cancers. The inactivation of these genes is an important step in transforming the host cell into one that supports replication of the adenoviruses. How would you engineer an adenovirus to kill cancerous cells possessing mutated p53 proteins while leaving normal cells unharmed?
18.2Some cells are permissive with respect to a virus while others are not. In a permissive cell, the virus can gain entry by binding a receptor and once inside the cell the viruses can replicate themselves. In nonpermissive cells, there aren’t any receptors that can be exploited for entry, and if they do the signaling pathways do not support completion of the viral replication cycle.
One of the signaling pathways often altered in cancer cells is the Ras signaling pathway. One of the downstream targets in this growthpromoting pathway is the antiviral agent PKR. As mentioned earlier, when activated PKR shuts down proteins synthesis thereby preventing viral replication. Phosphorylation by one or more kinases acting downstream from Ras represses this activity. How would you engineer an oncolytic virus to kill cancerous cells possessing mutated Ras proteins while leaving normal cells unharmed? Note: dsRNA viruses often encode an anti-PKR protein.

19
Ion Channels
The plasma membranes of nerve and muscle cells differ from those of other cells in the body. Whereas the potential across the plasma membrane of any cell will change when the ion permeability across the membrane is altered, only in nerve and muscle cells will the converse occur. That is, in nerve and muscle cells, the ion permeability will change as a result of a change in membrane potential. This property is a consequence of the presence in the plasma membrane of large numbers of ion channels that open and close in response to changes in voltage. When an ion channel opens, up to 10 million ions can flow though its conducting pore per second, generating a few picoamperes (10-12 amps) of current. The presence of a few thousand of these channels endows the plasma membrane with the ability to actively conduct action potentials. Ion channels are responsible for all electrical signaling, and regulate a variety of processes including osmotic balance, hormone release, heartbeat, motor movement, learning, and memory.
Ion channels render the plasma membrane permeable to the passage of certain inorganic cations (Na+, K+, Ca2+) and anions (Cl-). The channels are composed of several subunits arranged in a way that creates a hollow pore that permits the one-way passive diffusion of ions from either outside the cell in, or inside the cell out. Some channels permit the passage of sodium, while others allow potassium ions or calcium ions or chloride ions to pass through. Ion channels are not open all the time, but rather open and close in response to extracellular ligand (neurotransmitter) binding, intracellular ligand binding, or to changes in membrane voltage. They possess one or more “gates” that mechanically open and close their pores to the passage of ions in response to the regulatory signals.
The first part of the chapter is devoted to an examination of how membrane potentials arise and how action potential can be generated from voltage-dependent changes in the permeability of the plasma membrane. A number of key biophysical concepts such as channel gating, and voltagedependent activation and deactivation will be introduced along with the Nernst and Hodgkin–Huxley equations. The second part of the chapter is dedicated to the mechanisms that make properties such as gating possible.
465

466 19. Ion Channels
These mechanisms are revealed by electron microscopy and X-ray crystallography studies of the atomic structure of the ion channel proteins.
19.1 How Membrane Potentials Arise
Membrane potentials arise from ion concentration differences and selective permeability. Nerve and muscle cells, unlike other cell types in the body, possess appreciable membrane potentials or membrane potential differences. Their membrane potentials arise from differences in ionic concentrations between outside and inside environments and from the selective permeability of the membrane to specific ion species due to the presence of ion channels. To see how ion concentrations and selective permeability give rise to membrane potentials four ion species will be considered— sodium (Na+), potassium (K+), chloride (Cl-), and A-. The A- ion species represents the net negative charge of the large biomolecules residing within the cell. The four ion species are distributed outside and inside the cell subject to two conditions:
•Osmotic balance—The net concentration of ions outside the cell must equal the net concentration of ions inside the cell.
•Charge balance—The net charge both outside the cell and inside the cell must be zero.
The first condition ensured that the cell will not be subject to osmotic stresses and the second condition is a charge equalization requirement in each compartment. The concentrations of these four ion species for a representative nerve cell are depicted in Figure 19.1(a). As can be seen by
FIGURE 19.1. Ion concentrations inside and outside the cell and membrane permeability: (a) Inside and outside are separated by an impermeable membrane represented by the double dashed lines. (b) The membrane separating the two compartments contains ion channels and is permeable to passage of potassium and chloride ions into the cell and sodium ions out of the cell. Ion concentrations are shown in units of millimoles per liter (mM/liter or mM).

19.1 How Membrane Potentials Arise |
467 |
adding up the concentrations the osmotic and charge conditions |
are |
satisfied. |
|
The membrane separating inside and outside in part (a) of the figure is impermeable to the passage of ions. Since there is no membrane permeability there is no membrane potential. Consider now what happens when ion channels are added as in part (b) of Figure 19.1. Channels for K+, Na+, and Cl- ions are now present, but not for A- ions, which are too large to pass through an ion channel. The sodium and chloride channels will be neglected for the moment, with the main attention on the K+ and the A- ion concentration.
Potassium channels allow potassium ions to passively diffuse out of the cell. They will be driven to do so by the potassium concentration gradient set up because the potassium concentrations outside and inside the cell differ. As potassium ions diffuse out of the cell a net negative charge will build up within the cell since the negative charge associated with the A- ions exceeds the positive charge of the remaining potassium ions. The buildup of negative charge will oppose the outward flow of positively charged potassium ions, eventually reaching the point where it is strong enough to halt the flow of ions. At this point an equilibrium situation is reached. There is a potential difference across the membrane and this potential just balances the concentration gradient. The potential difference required to halt the flow of potassium ions through the potassium channels is the potassium membrane potential, similar to the situation for other ion species. These potentials are given by the Nernst equation:
EX = |
RT |
[X]out |
|
||
|
ln |
|
. |
(19.1) |
|
zF |
[X]in |
||||
In the Nernst equation, R is the universal gas constant, T is the absolute temperature, F is Faraday’s constant, z is the valence of the ion (+1 for sodium and potassium; +2 for calcium, and -1 for chloride), and X stands for the ion species under consideration. At a temperature of 37° Celsius, RT/F = 26.73 mV, and switching from natural logarithms to base 10 logarithms:
EX = |
61.54 |
|
[X]out |
(19.2) |
|
|
log |
|
. |
||
z |
[X]in |
||||
The potentials given by the Nernst equation are commonly referred to as reversal potentials, or alternatively, as equilibrium potentials. They can be either positive or negative, depending on the sign of z and on which concentration is greater—inside or outside. As noted in Table 19.1, the reversal potentials for sodium and calcium are positive while those for potassium and chloride are negative.
The plasma membrane of cells is more permeable to some ions than to others. It is far more permeable to potassium ions than to sodium ions, and

468 19. Ion Channels
TABLE 19.1. Values for typical concentrations of four main kinds of ions inside and outside mammalian skeletal muscle cells are presented along with the values for the equilibrium potential calculated from the Nernst equation.
Ion species |
[X]out(mM) |
[X]in(mM) |
Ex(mV) |
Na+ |
145 |
12 |
+67 |
K+ |
4 |
155 |
-98 |
Cl- |
123 |
4 |
-92 |
Ca2+ |
1.5 |
10-4 |
+129 |
somewhat more permeable to potassium ions than to chloride ions. The resting potential of a membrane (Vm) is given by combining the currents from the various ion species, taking into account not only the concentrations outside and inside but also the relative permeabilities. A simple expression, known as the Goldman–Hodgkin–Katz equation, incorporates both of these factors:
|
K |
]out |
+ (pNa |
pk ) |
Na |
]out |
+ (pCl |
pk ) Cl |
]in |
|
|
|||||
Vm = 61.54 log |
[ |
|
|
[ |
|
|
[ |
|
. |
(19.3) |
||||||
[ |
K |
]in |
+ (pNa |
pk ) |
Na |
]in |
+ (pCl |
pk ) Cl |
]out |
|||||||
|
|
|
|
[ |
|
|
|
[ |
|
|
||||||
As is customary, the calcium contribution, which is small, has been neglected. In Eq. 19.3, pK, pNa and pCl are permeability constants. The appearance of their ratios in Eq. 19.3 takes into account the relative permeabilities of the membrane to the different ion species. Typical resting potentials are in the range -60 to -80 mV. These values are far closer to the potassium equilibrium potential than to the sodium equilibrium potential. The closeness of the membrane resting potential to the potassium reversal potential is a reflection of a far greater permeability for potassium than for either sodium or chloride. As their permeability ratios in the GHK equation become negligible, the expression for the resting potential approaches that of the Nernst equation for potassium. The reversal potential may be thought of as supplying a driving force for changes in resting potential.
19.2 Membrane and Action Potentials Have
Regenerative Properties
Action potentials are generated by the joint action of fast-acting sodium channels and delayed-acting potassium channels. The generation of an action potential begins with a threshold-exceeding depolarization that opens sodium channels. When this happens the permeability of the membrane to sodium increases; according to the GHK equation the membrane depolarizes as its potential moves towards the equilibrium value for sodium. The open sodium channels permit the entry of sodium cations into the cell, which depolarize the membrane even further allowing for an
19.2 Membrane and Action Potentials Have Regenerative Properties |
469 |
opening of still more sodium channels. This continues for only a short while. The positive feedback of sodium channel openings stimulating the further opening of sodium channels is terminated when the channels inactivate.
At this point, the potassium channels, which also open in response to depolarization, start to dominate. The buildup of positive charge inside the cell through the entry of sodium cations is now countered by a decrease in positive charge through the exit of potassium cations. This slower activity moves the membrane potential towards the reversal potential for potassium ions. The opening of potassium channels continues for some time resulting in the repolarization of the membrane, and contributing to the shut down of the sodium channels. At the start of the cycle the resting potential is about -60 mV. The membrane depolarizes to the extent that its potential reaches positive values due to the sodium influx. It repolarizes to hyperpolarized values of more than -70 mV driven by the potassium efflux and then relaxes back to its resting potential. The hyperpolarization stage is important. It is needed to relieve the inactivation of the sodium channels, and prepare the membrane for the next round of activation and action potential generation.
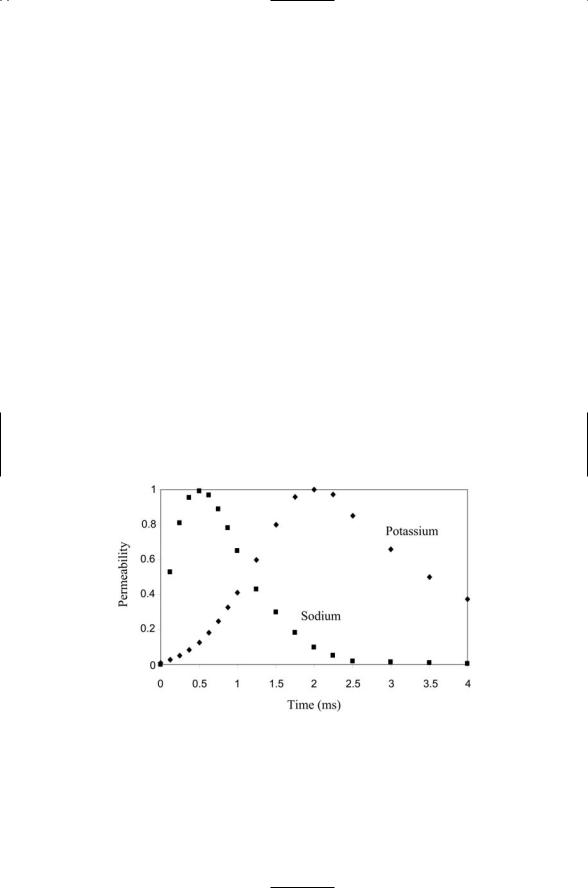
As shown in Figure 19.2, the time course of the flow of sodium ions through their channels differs from that of potassium ions through their channels. Sodium and potassium channels are both activated by depolarization, but the sodium channels open for a short time and then shut down
FIGURE 19.2. Time courses of sodium and potassium currents through a plasma membrane: Plotted are relative permeability values for the two ion species, that is, the number of open channels per unit surface area as a fraction of the peak number open channels/unit area for each ion species. Time is measured from initiation of an action potential by a threshold exceeding depolarization.

470 19. Ion Channels
even if the membrane potential has not changed from its depolarized value. That is, the sodium channels inactivate after being activated for a short time. Potassium channels activate more slowly than sodium channels, reaching their peak conductivity values after the sodium channels shut down. However, once they reach their peak conductivity values, the potassium channels remain open for some time. That is, potassium channels are noninactivating.
To summarize, sodium channels launch and generate the rising part of the action potential; positive feedback sustains it, and potassium channels generate the slowly declining following portion of the pulse. The positive feedback loop of the sodium channels is the key to generation of action potentials that propagate down axons without diminishing. Once a small depolarization is started in a limited region of space, the spread of ions laterally to neighboring spaces triggers a growing depolarization in that space and from there to the next neighboring space and so on. The pulse does not die out since a small depolarization triggers a large one, that is, the signal is amplified, as a result of the positive feedback. Thus, once started, a pulse propagates along without losing strength, that is, the pulses are regenerative. The existence of a threshold all-or-nothing response is a consequence of the positive feedback from the sodium channels and negative feedback from the potassium channels. Negative feedback is present because the depolarization-induced exit of potassium hyperpolarizes the membrane, thereby acting negatively to shut down the potassium ion flow. As a result of both forms of feedback there is an all-or-nothing response. It takes a certain amount of depolarization to trigger the positive feedback dominated sodium influx, and once started the process runs to completion.
19.3 Hodgkin–Huxley Equations Describe How Action
Potentials Arise
In 1952, Alan Hodgkin and Andrew Huxley constructed a mathematical model of the time course of sodium and potassium permeability changes in the giant axon of the squid. There are two ways to preserve and speed up the conduction of electrical pulses over long distances in an axon: either by increasing the diameter or by adding an insulating fatty sheath (myelination). The squid giant axon is unmyelinated and makes use of the first of the two solutions to the conduction problem. It is enormous, with a diameter that can reach 1 mm. It can be easily manipulated in the laboratory, and is thus is an ideal system for laboratory studies of how pulses are transmitted down nerves. The Hodgkin–Huxley model represents the culmination of decades of study by Hodgkin, Huxley, Katz, and many others on how electrical pulses are produced, and how they propagate, in nerve tissue. The development of the model was preceded by their detailed experimental studies of the flow of electrical current through the surface membrane of

19.3 Hodgkin–Huxley Equations Describe How Action Potentials Arise |
471 |
the squid giant axon. In these studies, a method, the “voltage clamp,” was developed that allowed researchers to directly measure current flow across the membrane of the axon.
The basic elements of the Hodgkin–Huxley model are a set of ionic currents separated into distinct contributions from sodium and potassium ions, and from the leakage current, representing the fairly constant and voltageindependent, background leakage of ions through the plasma membrane. These three currents are related to membrane permeabilities, which are expressed in terms of ionic conductances gNa, gK and gL, and the displacements of the membrane potential from the reversal potentials for the ion species. The relationship between current, conductance, membrane potential, and reversal potential is from Ohm’s law,
Ix = gx (Vm - Ex ). |
(19.4) |
In the above, X denotes the ion species under consideration, Vm the membrane potential, Ix is its current, Ex is its reversal potential, and gx is its conductance, the inverse of its resistance Rx, that is,
gx = 1 Rx . |
(19.5) |
In elementary circuits, R is a constant, and Ohm’s law says that current and voltage are linearly related. This is not the case for a biological membrane. Although the linear form of Ohm’s law is retained in the Hodgkin–Huxley equations, the sodium and potassium conductance are no longer constants, but instead are treated as functions of the membrane voltage.
Because of the nonconducting nature of the lipid bilayer the plasma membrane can be thought of as a capacitor that permits a buildup of charge on its surfaces. If a current is applied part will contribute to charging up the plasma membrane and part will flow through the membrane into the cell. The net ionic current is the sum of the sodium, potassium, and leakage currents plus any external applied or synaptic currents. The capacitive current
IC is, from Faraday’s law,
IC = C dVm . |
(19.6) |
dt |
|
In this expression, C is the capacitance, and dVm/dt is the time rate of change in the voltage. The membrane voltage does not instantaneously adapt to the flow of charge in and out of the cell. Instead, it takes a certain amount of time for charge to build up on the membrane and the voltage to adjust to the current flow. The complete circuit equation, expressing the conservation of charge, is
C dVm = -gK (Vm - EK ) - gNa (Vm - ENa ) - gL (Vm - EL ) + I , (19.7)
dt
where I is any applied or synaptic current and EL is leakage current.
An equivalent circuit for the membrane and its ion channels is presented in Figure 19.3. The Hodgkin–Huxley equation describes an electrical circuit
