
Molecular and Cellular Signaling - Martin Beckerman
.pdf
12.8 Hormone-Sending and Receiving Glands |
287 |
TABLE 12.4. Hormones secreted by the hypothalamus, adrenal, anterior pituitary, and endocrine pancreas glands: Listed are the names and either common abbreviations or alternative names of the hormones.
Hypothalamus |
Anterior pituitary |
Corticotropin-releasing hormone (CRH) Gonadotropin-releasing hormone (GRH) Growth hormone-inhibiting hormone (GHIH) Growth hormone-releasing hormone (GHRH) Prolactin-inhibiting hormone (PIH) Prolactin-releasing hormone (PRH) Thyrotropin-releasing hormone (TRH)
Adrenocorticotropic hormone (ACTH) Follicle-stimulating hormone (FSH) Growth hormone (GH)
Lutenizing hormone (LH) Melanocyte-stimulating hormone (MSH) Prolactin (PRL)
Thyroid-stimulating hormone (TSH)
Adrenal gland |
Endocrine pancreas |
Adrenaline (Epinephrin) |
Glucagon |
Noradrenaline (Norepinephrin) |
Insulin |
Steroid hormones |
Somatostatin |
adipose tissue, heart muscle, skeletal muscle, brain, and lungs. The adrenal medulla is surrounded by the adrenal cortex, a second distinct structure in the adrenal gland. The adrenal cortex releases a large number of steroid hormones the most important being aldosterone and cortisol that help maintain salt balance and water homeostasis in the body.
As mentioned previously, steroid hormones do not bind to receptors on the cell surface but instead pass through the plasma membrane and bind to intracellular (nuclear) receptors. Peptide hormones stimulate the synthesis and release of these hormones. Angiotensin II and III produced in the kidneys stimulate aldosterone production, ACTH stimulates cortisol production, and the glycoprotein hormone LH stimulates the release of the gonadotropic steroid hormones progesterone and testosterone.
Endocrine pancreas. The insulin molecule, like ACTH and its siblings, is generated from a precursor molecule, or prohormone. The finished insulin molecule, consisting of two chains for a total length of 51 amino acids, is produced by cleavage from a single chain precursor, proinsulin. Once secreted by beta cells of the Islets of Langerhans in the pancreas, insulin binds to receptor tyrosine kinases on target fat, muscle, and red blood cells. Glucogon, a 29-amino acid protein, is produced by alpha cells of the islets and by cells distributed throughout the gastrointestinal tract. It binds to a GPCR. A third hormone, somatostatin is produced by cells in the islets as well as by cells in the hypothalamus. All three of these hormones, insulin, glucagons, and somatostatin, influence the flow of nutrients in the body, and maintain glucose homeostasis by regulating the storage of glucose and its transport in and out tissues. Insulin release is stimulated by high blood sugar levels and triggers the uptake of glucose by its target cells. Glucagon works in the opposite direction. Glucagon receptors are found in the liver.

288 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
Glucagon is secreted by the pancreas in response to low blood sugar levels and signals the liver to make and secrete glucose.
12.9 Functions of Signaling Molecules
Signaling molecules are often used in more than one way. Some function as hormones and also as neuromodulators, substances that modify the electrical properties (excitability) of neurons. Other substances function both as neuromodulators and as neurotransmitters. The distinction between functioning as a hormone or as a neurotransmitter or as a neuromodulator is based on how the signaling molecule is being used rather than on its chemical composition.
Neurotransmitters are secreted in a highly directional manner. They are released from the pre-synaptic terminal of a neuron and diffuse across the synaptic cleft to the postsynaptic terminal of a neighboring neuron. There are two kinds of receptors in the nervous system for neurotransmitters, ionotropic and metabotropic. Ionotropic receptors are referred to as ion channels. They respond to a binding event by transiently opening a channel, an aqueous pore through the plasma membrane for the passage of ions— usually Na+, K+, Ca2+ or Cl-, depending on the type of neurotransmitter and receptor—in and out of the cell. This type of signaling is rapid and direct. Signaling through metabotropic receptors, another term for GPCRs, is slower and less direct.
Some of the most prominent neurotransmitters can signal through both ionotropic and metabotropic receptors. Glutamate and g-aminobutyric acid (GABA) are two of the most often encountered neurotransmitters in the brain. There are ionotropic glutamate receptors and metabotropic glutamate receptors. Similarly there are ionotropic GABA receptors and metabotropic GABA receptors; the former are referred to as GABAA receptors and the latter as GABAB receptors. Another prominent neurotransmitter is acetylcholine. This neurotransmitter can bind to ionotropic (nicotinic) receptors and to G protein-coupled (muscarinic) receptors.
The four most common neurotransmitters found in the brain are listed in Table 12.5. The terms excitatory and inhibitory refer to the effects the neuro-
TABLE 12.5. Excitatory and inhibitory neurotransmitters used in the central nervous system: Common abbreviations for the neurotransmitters are shown in parentheses.
Excitatory |
Inhibitory |
Acetylcholine (ACh) |
g-Aminobutyric Acid (GABA) |
Glutamate (Glu) |
Glycine (Gly) |

12.10 Neuromodulators Influence Emotions, Cognition, and Pain |
289 |
transmitters have on membrane excitability. Inhibitory neurotransmitters bind to anionic ion channels and decrease excitability, while excitatory neurotransmitters bind to cationic ion channels and increase excitability. The subject of electrical excitability will be explored in Chapter 19.
Neuromodulators are secreted in a broader manner than neurotransmitters, and this kind of release allows them to influence a large number of cells. They bind to GPCRs and modify the excitability of the target cells by regulating the activities of their ion channels mostly through inhibitory mechanisms. Neuromodulators acting through GPCRs provide a means whereby information from cells influenced by a population of excitable cells can be fed back to the originating population thereby regulating their electrical activities.
There are several routes of G protein regulation of ion channels. There is a direct one where Ga and Gbg subunits directly bind and regulate ion channels, and a number of indirect ones that operate through the second messenger-binding and second messenger-activated protein kinases. The direct route provides an efficient means for the regulation of electrical activity in excitable cells by chemical messages since GPCRs, G proteins, and ion channels are all membrane associated. A striking example of this form of regulation is the gating of cardiac potassium channels by Gbg subunits. The specific K+ channels regulated in this manner are known as G protein-linked inward rectified K+ channels (GIRKs). Binding of acetylcholine to the muscarinic (acetylcholine) GPCR leads to the activation of the GIRKs by Gbg subunits in cardiac pacemaker cells producing a slowing of the heart rate. This process is not restricted to the heart; GIRKs are also found in the brain and pancreas. A classic example of indirect regulation is phototransduction by rhodopsin and its retinal ligand.
The most common form of neuromodulation is through the indirect, second messenger-mediated regulation of ion channels. In this process, Gs,
Gi, and Gq alpha subunits stimulate or suppress second messengers and second messenger-dependent protein kinases that phosphorylate Ca2+, Na+, and K+ channels, thereby leading to changes in the ion channels’ structural and kinetic properties. Because of the amplification inherent in second messenger systems the neuromodulatory signals are able to modulate the activities of many ion channels at the same time. This indirect method adjusts the overall firing properties of the excitable cells, and in many cases alters the way the neural circuit operates.
12.10Neuromodulators Influence Emotions, Cognition, Pain, and Feeling Well
Neuromodulators produce changes in the electrical excitability of neurons and neural circuits that can last for hours and days. Some neuromodulators are peptides; others are nucleotides or amines. When acting in the brain

290 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
TABLE 12.6. Different categories of neuromodulators: The neuromodulators are classified according to their chemical derivation.
Amines |
Peptides |
Adrenaline |
Angiotensins |
Dopamine |
Neurokinins |
Histamine |
Oxytocin |
Noradrenaline |
Substance P |
Serotonin |
Vasopressin |
|
|
Nucleotides |
Endogenous opioids |
|
|
Adenosine |
Dynorphins |
ADP, ATP |
Endorphins |
GDP, GTP |
Enkephalins |
|
|
these neuromodulators influence cognition and emotions. Listed in Table 12.6 are a number of the most prominent neuromodulators that act in the brain, such as adenosine, serotonin, and dopamine. Serotonin is distributed at many places in the brain. It is synthesized from the amino acid L- tryptophan, and it is often referred to as 5-hydroxytryptamine (5-HT). Another amine—histamine—was discussed earlier as a mediator of inflammatory responses in the immune system. When secreted by mast cells it can bind to receptors expressed on peripheral nerve endings and serve as a neurmodulator.
Endorphins and tachykinins are examples of peptide neuromodulators. Endorphins—endogenous morphine—along with many other opiates influence perceptions of pain and pleasure. These substances bind to the opioid family of GPCRs expressed on the surface of neurons in the brain and spinal cord. Neuromodulators binding to opioid receptors have been placed under a separate heading in Table 12.6 reflecting their binding properties and actions. Endorphins have analgesic properties, and are also involved in maintaining water balance and other endocrine functions. Members of the endorphinlike family of opiates include beta-endorphin, enkephalins, and dynorphins. Beta-endorphins are synthesized in the pituitary as mentioned earlier, and enkephalins are produced in the adrenal medulla by chromaffin cells. These neuromodulators along with dynorphins are broadly distributed in several specific areas of the brain and spinal cord where they bind to their cognate opioid receptors. The tachykinins are another family of neuromodulators involved in the perception of pain. Mammalian members of this family, the neurokinins, include substance P (SP), neurokinin A (NKA) and neurokinin B (NKB).
Changes in the amount of neuromodulators in sensitive parts of the body produce sensations leading to alterations in behavior. The angiotensins are a family of regulators of blood pressure, blood volume, and cardiac vascu-

12.11 Ill Effects of Improper Dopamine Levels |
291 |
lar function. Production of angiotensins is stimulated by angiotensinconverting enzyme (ACE) and renin, a glycoprotein hormone produced by the kidney and other places in the body including the brain. An infusion of angiotensin II, the primary active angiotensin, into the brain of mammalian test subjects triggers the sensation of thirst and a craving for sodium (salt). Laboratory animals stop what they are doing and immediately begin drinking, and they exhibit an increased appetite for sodium when so infused.
12.11 Ill Effects of Improper Dopamine Levels
As mentioned earlier, G-protein coupled receptors and the signaling molecules that activate them are involved in a host of neurological disorders and are principal target of therapeutic drugs. Dopamergic neurons are those neurons that synthesize and secrete dopamine. Improper dopamine levels contribute to Parkinson’s disease, ADHD, schizophrenia, and other disorders. There are three main groups of dopamine secreting cells: one group located in an area of the midbrain called the substantia nigra (SN), another in the ventral tegmental area (VTA), and a third in the hypothalamic nuclei. These cells, along with several other smaller groups of dopamine-secreting cells, are referred to as the dopamine system. This system of cells is involved in drug addiction, Parkinson’s disease, Tourette syndrome, schizophrenia, and attention-deficit hyperactivity disorder (ADHD). In Parkinson’s disease, cells in the SN that secrete dopamine die leading to a deficiency in dopamine in the brain. In schizophrenia, antagonists, drugs that inhibit dopamine signaling, are used to suppress an overactive dopamine system. The most common feature of drug addiction is an elevation in dopamine signaling.
Cells in these three areas send out dopamergic signals to a large number of brain areas. The cells receiving these signals express two kinds of dopamine receptors. Receptors of the D1 type (D1 and D5) act through Gs subunits to activate adenylyl cyclase. Receptors of the D2 type (D2, D3, and D4) exert their influences through Gi subunits to suppress adenylyl cyclase and activate potassium channels.
Children and adults with ADHD exhibit behavior that is inattentive, impulsive, and hyperactive. In many of these patients there is reduction in size of the basal ganglia and frontal lobe responsible for controlling these behavioral responses. It appears that the level of dopamine signaling is too low, either because of the presence of too many dopamine transporters that remove dopamine from the extracellular milieu, or because of inadequate amounts of dopamine released from presynaptic terminals. These deficits are countered through the application of drugs such as methylphenidate [Ritalin®] resulting in increased levels of extracellular dopamine in the brain.

292 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
12.12Inadequate Serotonin Levels Underlie Mood Disorders
The serotonergic system has, as its serotonin-producing core, cells in the midbrain and pons close to where the brain and spinal cord join. The serotonin-producing cells are organized into clusters called Raphe nuclei. The neurons in the nuclei are large and send out their processes to almost every locale in the brain. Serotonin receptors can be grouped into seven classes, designated as 5-HT1 through 5-HT7. All but the 5-HT3 receptors are GPCRs. The 5-HT3 receptors are ligand-gated ion channels (discussed in Chapter 19). The best-studied GPCR classes are the 5-HT1 and 5-HT2 receptors. Members of the 5-HT1 grouping act through Gi and G0 types of G-proteins alpha subunits. They inhibit adenylyl cyclase activity and stimulate the opening of hyperpolarization-producing potassium channels. Serotonin 5-HT2 receptors work through Gq subunits to increase IP3 and intracellular calcium levels, and depolarize neurons by closing potassium channels.
Inadequate serotonin levels are responsible in a variety of mood disorders. Serotonin levels are increased in several ways to treat depression and panic attacks as well as other anxiety disorders. Drugs that increase serotonin levels by inhibiting the reuptake of serotonin are especially popular. Prozac® (fluoxitine) is a selective serotonin reuptake inhibitor (SSRI), as are other popular drugs such as Paxil® and Zoloft®. Serotonin and noradrenaline are monoamines. Another class of drugs that elevate serotonin levels is the monoamine oxidase inhibitors, or MAOIs. Monoamine oxidase is an enzyme that degrades serotonin and noradrenaline. The MAOIs work by inhibiting the actions of monoamine oxidase. Yet another group of serotonin-increasing drugs are the tricyclic antidepressants (TCAs). These too prevent serotonin reuptake.
12.13GPCRs’ Role in the Somatosensory System Responsible for Sense of Touch and Nociception
G protein-coupled receptors play a central role in the sensing and transmission of messages of a warning nature in the somatosensory system. The somatosensory system handles four categories of touch information: proprioception, simple touch (pressure, texture, and vibration sensations), temperature (heat sensations), and pain. The term “proprioception” refers to body and body part position, orientation, and location. This faculty utilizes mechanoreceptors that sense forces in muscles, tendons, and joints. Simple touches such as pressure are sensed by special receptors in the skin. Sensations of a warning nature—of temperature and pain—are mediated by free peripheral nerve endings, called nociceptors, which extend through-

12.14 Substances that Regulate Pain and Fever Responses |
293 |
TABLE 12.7. Hormonal signaling during injury and pain.
Local hormone
Adenosine
ADP, ATP
Bradykinin
Histamine
Prostanoids
Serotonin
Substance P
out the body and use a variety of G protein-coupled receptors and ion channels to sense and transduce signals.
Nociceptors are sensitive to extracellular chemical, mechanical, and thermal environments. In response to inappropriate conditions, they transmit signals through spinal neurons to brain receptors resulting in the perception of pain. The “pain pathway” is the general term given to the signaling route from the peripheral neurons located in places such as the skin to the spinal cord to the brain. Nociceptors are distributed throughout the body, but are generally absent in the brain, deep tissues, and visceral organs such as the liver, spleen, and lungs. The cell bodies of the free nerves are located in dorsal root ganglia (DRG). Signals travel from the DRG to the dorsal horn of the spinal cord and from there to the brain. There are two kinds of free nerve fibers, Ad and C. Ad fibers are thin and myelinated, and rapidly conduct sharp pain signals. C fibers are unmyelinated and slowly conduct aching, itching, and burning signals. The C fibers contain receptors for chemical signals sent by injured cells and by cells of the immune system. (Note: Myelinated fibers are fibers, or axons, that are sheathed in myelin, a fatty protein material that insulates the axons and promotes fast conduction of nerve pulses.)
A variety of chemical signals associated with injury and inflammation are exchanged between the nervous and immune systems. Injured cells release some of these warning chemicals, and cells of the immune systems such as mast cells secrete others. These messages (Table 12.7) act as local hormones that bind to receptors on peripheral nerve cells. Some of these signaling molecules and their receptors were discussed earlier. Those not yet examined include prostanoids, bradykinin, and purines such as adenosine. These are discussed next.
12.14Substances that Regulate Pain and Fever Responses
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been relied on for reducing inflammatory responses including pain and fever for 3500 years. Early medicinal extracts from willow bark led to the first commercial
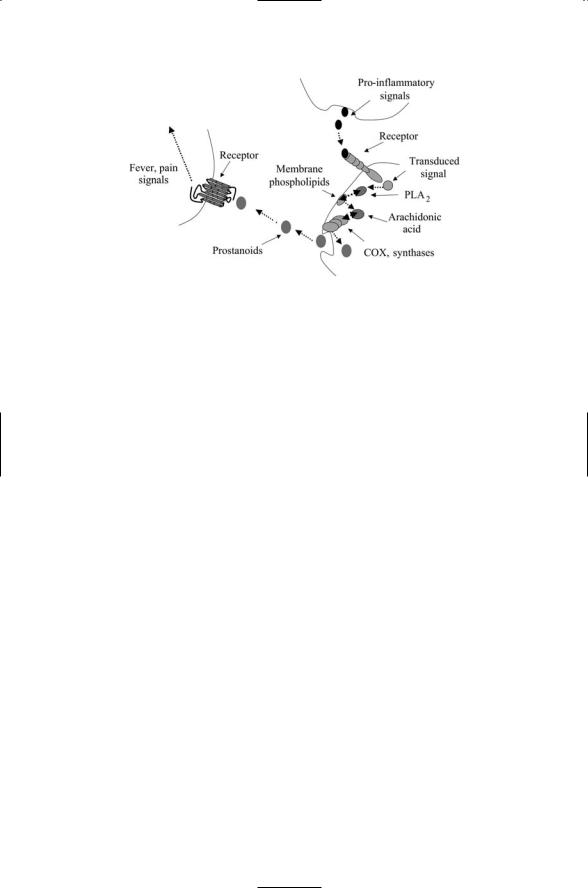
294 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
FIGURE 12.7. Generation of fever and pain signals: Pro-inflammatory signals activate phospholipase A2, which hydrolyzes membrane phospholipids (here depicted as phospholipids in the plasma membrane) resulting in arachidonic acid. A sequence of enzymes, beginning with COX, produces prostanoids from the arachidonic acid. The prostanoids are short lived with half lives on the order of a minute or so and are immediately secreted from the cell. Prostanoid receptors on peripheral nerve endings bind the prostanoids, and the neurons convey the pain and fever signals to the brain.
product aspirin one hundred years ago and to a host of more recent NSAIDs such as ibuprofen and naproxen. All of the NSAIDs work the same way—they inhibit the catalytic actions of a set of enzymes variously called endoperoxide H synthases (PGHSs) or cyclo-oxygenases (COXs). Two COX isoforms are targeted by anti-inflammatory NSAIDs. The first of these, COX-1, is constitutively active and is found primarily in the stomach and kidneys. The second, COX-2, is induced in many different cell types in response to inflammatory signals conveyed by cytokines, growth factors, and hormones. Inhibition of COX-1 can be harmful and the aim in drug design is to preferentially target the COX-2 isoform.
Cells release prostanoids (prostaglandins) when injured by chemical, thermal, or mechanical agents. Prostanoids are fatty acid derivatives of membrane lipids. As depicted in Figure 12.7, the first step in their production is the hydrolysis of membrane lipids by phospholipase A2 (PLA2) resulting in the release of arachidonic acid (AA). The AA intermediate is then converted to prostanoids through the sequential actions of the COXs and several other enzymes. The catalytic activities of PLA2 are stepped up in response to the cytokine, growth factor, and hormonal signals. In response the cells produce and then secrete prostanoids. During the inflammatory response G protein-coupled prostanoid receptors expressed on peripheral neurons bind prostaglandin. Signals sent on to thermoregulators

12.15 Composition of Rhodopsin Photoreceptor |
295 |
in the brain trigger an increase in body temperature. Aspirin and the other NSAIDs act to lower temperature and reduce pain by inhibiting the COXs, thereby preventing formation of and signaling by the prostanoids. The prostanoid signals are conveyed in a paracrine fashion to their cellular targets. For this reason prostanoids and other hormones acting in a paracrine manner are termed “local hormones.”
Bradykinin is a nine-amino acid residue peptide rapidly produced after tissue injury occurs. It is a central element in the inflammatory response. It is involved in regulating blood flow (vascular dilation), increasing vascular permeability, smooth muscle contractions, stimulating the release of prostaglandins and other inflammatory mediators, and pain signaling. Bradykinins bind to two kinds of receptors, B1 and B2. B2 receptors are constitutive in neurons and smooth muscle cells; B1 receptors, in contrast, are rapidly upregulated when there is tissue injury. B1 and B2 receptors are found in free nerve endings and directly mediate pain signaling.
Adenosine, ATP, and ADP serve as neurotransmitters and neuromodulators in the central and peripheral nervous systems. When released into extracellular spaces these molecules bind to purine receptors. Purine receptors are divided into two families, P1 and P2, each containing a number of subtypes (Table 12.1). Adenosine attaches to P1 receptors while ATP, ADP, and the pyrimidines UTP and UDP bind to P2 receptors. Adenosine and the other nucleotides carry out a large number of signaling tasks in the body. In heart muscle, adenosine decreases heart rate and lowers blood pressure. Adenosine is associated with sleep; during sleep deprivation adenosine levels build up in the brain. When bound to adenosine receptors in the brain, adenosine decreases neural activity leading to sleep. Caffeine is structurally similar to adenosine, and is an adenosine antagonist acting on adenosine A1 and A2A receptors. Adenosine and ATP are released into the extracellular spaces during injury and inflammation. Mast cells, neutrophils, and free nerve endings express P1 and P2 receptors, and adenosine helps mediate communication between the immune and nervous systems, and contributes to the pain response.
12.15 Composition of Rhodopsin Photoreceptor
The rhodopsin photoreceptor is composed of an opsin GPCR and its ligand, 11-cis-retinal. G protein-coupled receptors function as sensors and transducers of information about the external and internal environments.
GPCRs are involved in sight, taste, smell, touch, proprioception, and pain.
Touch, proprioception, and pain were discussed in the previous sections. In the remaining sections of this chapter, the focus will be on how signals conveyed by exogenous ligands such as light are transduced into cellular responses. The starting point will be how light, or electromagnetic energy,
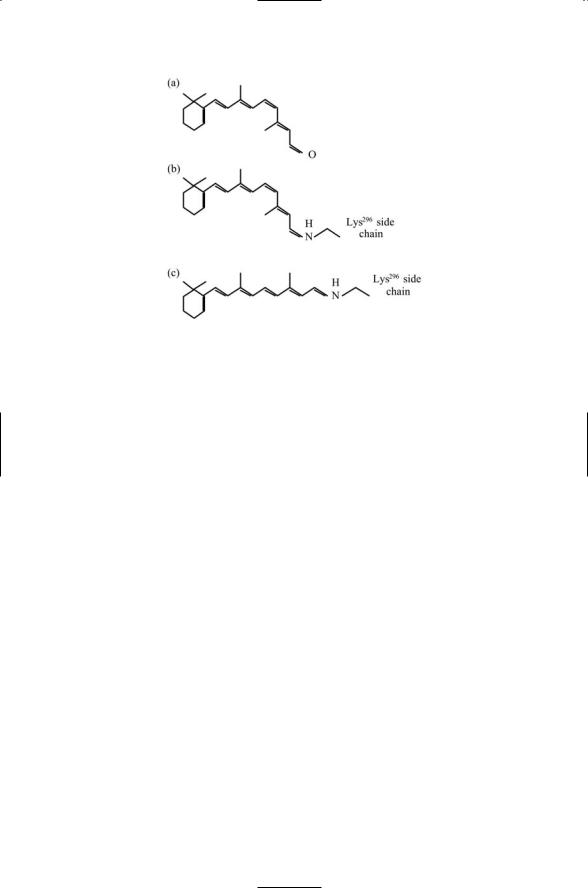
296 12. Signaling in the Endocrine and Nervous Systems Through GPCRs
FIGURE 12.8. Retinal photoisomerization: A Schiff base is an organic compound formed by the double bonding of a nitrogen atom of an amino group to a carbon atom. Schiff bases are formed in rhodopsin by the bonding of nitrogen of the NH3+ group on a lysine residue to retinal containing a CHO group with the accompanying release of a water molecule. Photoisomerization is the process whereby light impinges on and is absorbed by the double bonded structure resulting in the shifting and twisting of the bonds so that a number of atoms assume a different spatial orientation. (a) The retinal molecule. (b) Retinal in its 11-cis form covalently attached to a Lys296 side chain by a Schiff base. (c) The all-trans form of the covalently attached retinal molecule derived from the 11-cis form by photoisomerization.
striking a retinal molecule in converted into helix movements, a mechanical form of energy.
The retinal molecule, unlike most other ligands, is covalently linked to opsin and serves as a light-responsive chromophore. The retinal ligand is buried in a pocket that lies deep in the opsin protein. When a photon strikes the retinal chromophore it triggers a conformational change (retinal isomerization) from an 11-cis to an all-trans configuration, as shown in Figure 12.8. The retinal molecule is attached to the side chain of Lys296 situated in the H7 helix. The movement associated with the conformational change is appreciable—when stabilizing the alternative all-trans configuration there is a 4.5-Åmovement that is reflected in the positions of the cytoplasmic portions of the transmembrane helices. In its altered conformation rhodopsin activates the G-protein and initiates signaling in the vision pathway. The retinal is hydrolyzed and dissociated from the opsin after about a minute, enabling a new cycle of 11-cis-retinal attachment and activation.
