
Biomolecular Sensing Processing and Analysis - Rashid Bashir and Steve Wereley
.pdf114 |
NAM-TRUNG NGUYEN |
The usual procedure starts with in vitro tests, which can weed out the clearly dangerous, but may not expose all problems that may occur in the complex living system. In vitro tests should also be carried out to evaluate the adhesion behavior of cells, DNA, and other polymers. Furthermore, cytotoxicity of the device material should be tested for applications such as tissue engineering. Some animal tests may be required before the device moves to a human test stage. There are standardized tests to follow for the in vitro assessment.
In [40], typical materials of BMMDs such as metallic gold, silicon nitride, silicon dioxide, silicon, and SU-8 were evaluated using the cage implant system in mice. The materials were placed into stainless-steel cages and were sampled at 4, 7, 14, and 21 days, representative of the stages of the inflammatory response. The adherent cellular density of gold, silicon nitride, silicon dioxide, and SU-8TM were comparable and statistically less than silicon. These analyses identified the gold, silicon nitride, silicon dioxide, SU-8 as biocompatible with reduced biofouling.
5.6. CONCLUSIONS
Several fabrication issues were discussed in this chapter. BMMDs can be fabricated with polymeric micromachining technologies at a lower cost. Device examples in this article demonstrated, that BMMDs can be fully made of polymers. Polymers can work as substrate materials (section 5.3.1.2), sacrificial materials, and functional materials (section 5.3.2). Metals were successfully deposited on polymers using micromachined stencils (section 5.3.2.2). Polymeric BMMDs promise a simple design of interconnects (section 5.4.3) and a better degree of biocompatibility (section 5.5).
REFERENCES
[1]H. Becker and G¨artner. Electrophoresis, 21:12, 2000.
[2]D.J. Beebe, G.A. Mensing, and G.M. Walker. Annu. Rev. Biomed. Eng., 4:261, 2002.
[3]J. Black. Biological Performance of Materials. Marcel Dekker, New York, 1992.
[4]A. Braithwaite and F.J. Smith. Chromatographic Methods. 5. ed., Blackie Academic & Professional, London, 1996.
[5]M.G. Cowie. Polymers: Chemistry and Physics of Modern Materials. Intertext, Aylesbury, 1973.
[6]C.S. Effenhauser, A. Manz, and H.M. Widmer. Anal. Chem., 65:2637, 1993.
[7]V. Ghica and W. Glashauser (Verfahren fur¨ die spannungsfreie Entwicklung von bestrahlten Polymethyl- methacrylate-Schichten. German Patent, #3039110, 1982).
[8]C. Gonzalez, S.D. Collins, R.L. Smith. Sens. Actu. B, 49:40, 1998.
[9]D.J. Harrison, A. Manz, Z.H. Fan, H. Ludi,¨ and H.M. Widmer. Anal. Chem., 64:1926, 1992.
[10]A.C. Henry, T.J. Tutt, M. Galloway, Y.Y. Davidson, C.S. McWhorter, S.A. Soper, and R.L. McCarley. Anal. Chem., 72:5331, 2000.
[11]T.A. Horbett. Protein Adsorption on Biomaterials: Iterfacial Phenomena and Applications. American Chemical Society, Washington D.C., 2002.
[12]Q.A. Huang and N.K.S. Lee. J. Micromech. Microeng., 9:64, 1999.
[13]D.Hulsenberg¨ . Microelectron. J., 28:419, 1997.
[14]S.C. Jacobson, L.B. Kountny, R. Hergenroder,¨ A.W. Moore, and J.M. Ramsey. Anal. Chem., 66:3472, 1994.
[15]S.C. Jacobson, R. Hergenroder,¨ L.B. Coutny, and J.M. Ramsey. Anal. Chem., 66:2369, 1994.
[16]B.H. Jo et al. J. Micromech. Microeng., 9:76, 2000.
[17]H. Klank, J.P. Kutter, and O. Geschke. Lab on a Chip, 2:242, 2002.
[18]P. Krulevitch, W. Benett, J. Hamilton, M. Maghribi, and K. Rose. Biomed. Microdev., 4:301, 2002.
[19]C.H. Lin, G.B. Lee, Y.H. Lin, and G.L. Chang. J. Micromech. Microeng., 11:726, 2001.
FABRICATION ISSUES OF BIOMEDICAL MICRO DEVICES |
115 |
[20]A.P. London. Development and Test of a Microfabricated Bipropellant Rocket Engine. Ph.D. Thesis, Massachusetts Institute of Technology, 2000.
[21]H. Lorenz et al. J. Micromech. Microeng., 7:121, 1997.
[22]C. Luo, J. Garra, T. Schneider, R. White, J. Currie, and M. Paranjape. A New Method to Release SU-8 Structures Using Polystyrene for MEMS Applications. In Proceedings of the 16th European Conference on Solid-StateTranducers, 2002.
[23]M.J. Madou. Fundamentals of Microfabrication: The Science of Miniaturization. (2. ed., CRC Press, Boca Raton, 2002.
[24]J.C. McDonald, D.C. Duffy, J.R. Anderson, D.T. Chiu, H. Wu, O.J.A. Schueller, and G.M. Whitesides.
Electrophoresis, 21:27, 2000.
[25]E. Meng, S.Wu, and Y.-C. Tai. Micro Total Analysis System 2000. Kluwer Academic Publishers, Netherlands, 2000.
[26]N.T. Nguyen and S.T. Wereley. Fundametals and Applications of Microfluidics. Artech House, Boston, 2002.
[27]N.T. Nguyen, T.Q. Truong, K.K. Wong, S.S. Ho, L.N. Low. J. Micromech. Microeng., 14:69, 2003.
[28]D.L. Polla, A.G. Erdman,W.P. Robbins, D.T. Markus, J. Diaz-Diaz., R. Rizq, Y. Nam, H.T. Brickner, A. Wang, and P. Krulevitch. Annu. Rev. Biomed. Eng., 2:551, 2000.
[29]R. Salim, H. Wurmus, A. Harnisch, and D. Hulsenberg¨ . Microsys. Technol., 4:32, 1997.
[30]K. Seiler, D.J. Harrison, and A. Manz. Anal. Chem., 65:1481, 1993.
[31]S.A. Soper, S.M. Ford, S. Qi, R.L. McCarley, K. Kelley, and M.C. Murphy. Anal. Chem., 72:643A, 2000.
[32]G. Spierings. J. Mater. Sci., 28:6261, 1993.
[33]R. Srinivasan. Appl. Phys., 73:6, 1993.
[34]L.F. Thompson, C.G. Willson, and M.J. Bowden. Introduction to Microlithography. Americal Chemical Society, Washington D.C., 1994.
[35]T.Q. Truong and N.T. Nguyen. J. Micromech. Microeng., 14:632, 2004.
[36]W. Wijngarrt et al. The First Self-Priming and Bi-Directional Valve-Less Diffuser Micropump for Both Liquid and Gas. In Proceedings of MEMS’00 the 13th IEEE International Workshop on MEMS, 2000.
[37]A.T. Wolly and R.A. Mathies. Anal. Chem., 67:3676, 1995.
[38]Y. Xia and G.M. Whitesides. Annu. Rev. Mater. Sci., 28:153, 1998.
[39]T.J. Yao et al. Micromachined Rubber O-Ring Micro-Fluidic Couplers. In Proceedings of MEMS’00 the 13th IEEE International Workshop on MEMS, 2000.
[40]G. Voskericiana, M.S. Shivea, R.S. Shawgoc, H. von Recumd, J.M. Andersona, M.J. Cimac, and R. Langere. Biomaterials, 24:1959, 2003.
6
Intelligent Polymeric Networks
in Biomolecular Sensing
Nicholas A. Peppas1,2,3 and J. Zachary Hilt4
1 Center of Biomaterials, Drug Delivery, and Bionanotechnology Molecular Recognition, Department of Chemical Engineering
2 Department of Biomedical Engineering
3 Department of Pharmaceutics, The University of Texas, Austin, TX 78712-0231 U.S.A. 4 Department of Chemical and Materials Engineering, University of Kentucky, Lexington, KY 40506-0046 U.S.A.
Since the development of the first biological sensor over 40 years ago [1], the biosensor field has continuously evolved. Today, biosensors are applied in a wide range of uses, including environmental analysis, medical diagnostics, bioprocess monitoring, and biowarfare agent detection. The success of the biosensor is dependent on the ability to rapidly, sensitively, and selectively recognize various biomolecules, with relative importance dependent on the application.
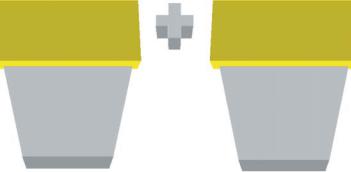
The tailored recognition of the desired biomolecule by the sensing element is the first step in the biosensing process, and the second step is the translation of the interaction into a measurable effect via the transduction element (Figure 6.1). In sensor platforms, a wide variety of transduction methods have been employed, such as gravimetric [2–5], optical [6, 7], and electrochemical [8] transducing elements.
For biosensor applications, the sensing element is typically natural bioreceptors, such as antibody/antigen, enzymes, nucleic acids/DNA, cellular structures/cells, due to their evolved high affinity and specificity [9, 10]. Biomimetic sensing elements, such as those based on polymer networks, can be advantageous over their biological counterparts because they can be designed to mimic biological recognition pathways and at the same time exhibit other abiotic properties that are more favorable, such as greater stability in harsh environments [11, 12].
118 NICHOLAS A. PEPPAS AND J. ZACHARY HILT
Recognition |
Transduction |
|
Element |
Element |
|
Cellular structures/cells |
Electrochemical |
|
Nucleic acids/DNA |
||
|
||
Antibody/antigen |
Gravimetric |
|
Biomimetic |
Optical |
|
Enzymes |
|
FIGURE 6.1. Illustration of the critical components of a biosensor device and some examples of each.
In particular, the development of microand nanoscale sensor platforms has greatly enhanced the applicability of the resultant devices. For instance, the field of clinical diagnostics presents numerous opportunities where microand nanoscale biosensor technology can be exploited [13, 14]. For successful patient treatment, medical diagnostics depends on the rapid and precise detection of signature biomolecules for a condition. With the development of lab-on-a-chip and other miniature point-of-care, the speed and precision with which health care is administered has been radically enhanced. These micro or miniaturized total analysis systems (µ-TAS) integrate microvalves, micropumps, microseparations, microsensors, and other components to create miniature systems capable of analysis that typically requires an entire laboratory of instruments. Since introduced as a novel concept for chemical sensing devices [15], reviews have been published that have focused on the application of µ-TAS as innovative point-of-care diagnostic [16, 17].
By developing microand nanoscale sensor elements and the corresponding sensor platforms, point-of-care diagnostic devices can be fabricated that not only have a significant impact in ex-vivo sensing applications, but can also be applied to in-vivo and in-vitro applications, where microor nanoscale dimensionality is imperative. These miniaturized sensors require small sample and/or reagent volumes, tend to be less invasive, and can be faster and more sensitive relative to macroscale technologies.
In this chapter, we point out how intelligent polymer networks can be used as fundamental sensing elements in biosensor devices focusing on the advancements towards microand nanoscale sensor platforms that will lead to improved and novel analysis and impact a wide variety of fields, including environmental analysis and medical diagnostics.
6.1. INTELLIGENT POLYMER NETWORKS
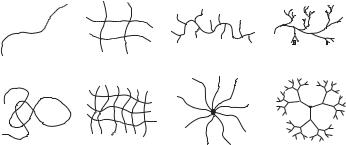
A polymer network is a three dimensional structure formed via physical or chemical crosslinking of polymer chains, creating one giant macromolecule where all monomer units are connected to each other within the polymer phase. The major classes of macromolecular structures are illustrated in Figure 6.2. Polymer networks are prepared by reacting functional monomers and crosslinkers beyond the critical extent of reaction, referred to as the gelation point, where the transition between free polymer chains and an insoluble polymer network
INTELLIGENT POLYMERIC NETWORKS IN BIOMOLECULAR SENSING |
119 |
|||
Linear |
Crosslinked |
Branched |
Dendritic |
|
Linear Chain Lightly Crosslinked Random-branched Hyperbranched
Flexible Coil |
Densely Crosslinked |
Star |
Dendrimer |
|
|
|
(Starburst) |
FIGURE 6.2. Illustration of the major classes of macromolecular architecture.
occurs. Various initiation mechanisms, using photochemical, thermal, and redox initiation, are commonly utilized to synthesize polymer networks.
By tailoring their molecular structure, polymer networks can be created that interact with their environment in a pre-programmed, intelligent manner. In biosensor applications, these networks can be advantageous relative to there biological counterparts, since they can be designed to recognize and respond to a biological entity or environmental condition, while exhibiting other properties more favorable for sensor applications, such enhanced stability. Intelligent polymer networks have been created based on a variety of polymer systems, including environmentally responsive hydrogels, biohybrid hydrogel networks, and biomolecularly imprinted polymers.
6.1.1. Hydrogels
Hydrogels are hydrophilic polymeric networks that swell in water or biological fluids without dissolving. The swelling characteristics are the result of crosslinks (tie-points or junctions), permanent entanglements, ionic interactions, or microcrystalline regions incorporating various chains [18–21]. They have been used in various biomedical applications including linings of artificial organs, contact lenses, and biosensors. In the last twenty years, hydrogels have been researched as prime materials for pharmaceutical applications, predominantly as carriers for delivery of drugs, peptides or proteins. They have been used to regulate drug release in reservoir-type, controlled release systems, or as carriers in swellable matrix systems [22].
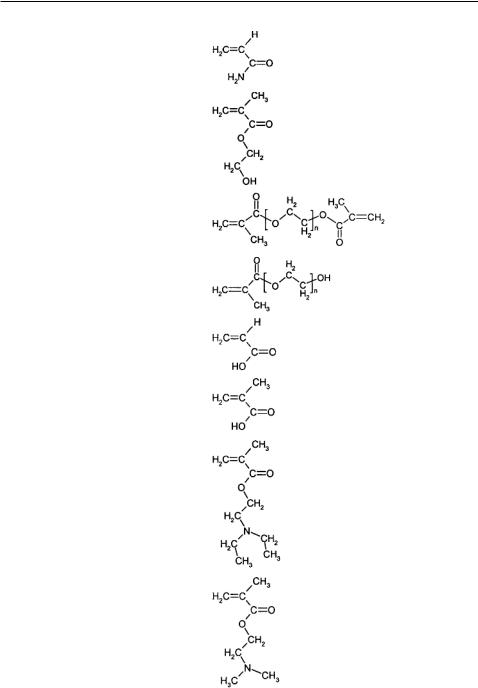
Hydrogels can be classified as neutral, anionic or cationic networks. In Table 6.1, some representative functional monomers and their relevant properties are listed for reference. The network swelling behavior is governed by a delicate balance between the thermodynamic polymer-water Gibbs free energy of mixing and the Gibbs free energy associated with the elastic nature of the polymer network. In ionic hydrogels, the swelling is governed by the thermodynamic mixing, elastic-retractive forces, and also by the ionic interactions between charged polymer chains and free ions. The overall swelling behavior and the associated response or recognition are affected by the osmotic force that develops as the
120 |
NICHOLAS A. PEPPAS AND J. ZACHARY HILT |
TABLE 6.1. Common functional monomers used in hydrogel synthesis.
Functional Monomer |
Abbrev. |
Structure |
Ionic Character |
|
|
|
|
Acrylamide |
AAm |
|
Neutral |
2-Hydroxyethyl |
2-HEMA |
Neutral |
methacrylate |
|
|
Poly(ethylene glycol)n |
PEGnDMA |
Neutral |
dimethacrylate |
|
|
Poly(ethylene glycol)n |
PEGnMA |
Neutral |
methacrylate |
|
|
Acrylic acid |
AA |
Anionic |
Methacrylic acid |
MAA |
Anionic |
2-(Diethylamino) ethyl |
DEAEMA |
Cationic |
methacrylate |
|
|
2-(Dimethylamino) ethyl |
DMAEMA |
Cationic |
methacrylate |
|
|
|
|
|
INTELLIGENT POLYMERIC NETWORKS IN BIOMOLECULAR SENSING |
121 |
charged groups on the polymer chains are neutralized by mobile counterions [23]. Electrostatic repulsion is also produced between fixed charges and mobile ions inside the gel, affecting the over swelling of the ionic gel. The equilibrium swelling ratios of ionic hydrogels are often an order of magnitude higher than those of neutral gels because of the presence of intermolecular interactions including coulombic, hydrogen-bonding, and polar forces [24].
6.1.2. Environmentally Responsive Hydrogels
There has recently been increased research in the preparation and characterization of materials that can intelligently respond to changing environmental conditions. By tailoring the functional groups along the polymer backbone, hydrogels can be made sensitive to the conditions of the surrounding environment, such as temperature, pH, electric field, or ionic strength. Because the actuation process is governed by water uptake, these hydrogel systems are attractive for any aqueous applications, and this has led to extensive research being focused on developing biosensor devices based on these hydrogels. Recent reviews have highlighted the extensive research focused on developing new and applying current environmentally sensitive hydrogels, specifically those sensitive to temperature, pH, and specific analytes [11, 25–27].
Certain hydrogels may exhibit environmental sensitivity due to the formation of polymer complexes. Polymer complexes are insoluble, macromolecular structures formed by the non-covalent association of polymers with the affinity for one another. The complexes form due to association of repeating units on different chains (interpolymer complexes) or on separate regions of the same chain (intrapolymer complexes).
Polymer complexes can be stereocomplexes, polyelectrolyte complexes, and hydrogen bonded complexes. The stability of the associations is dependent on such factors as the nature of the swelling agent, temperature, type of dissolution medium, pH and ionic strength, network composition and structure, and length of the interacting polymer chains. In this type of gel, complex formation results in the formation of physical crosslinks in the gel [28]. As the degree of effective crosslinking is increased, the network mesh size and degree of swelling is significantly reduced.
6.1.3. Temperature-Sensitive Hydrogels
Certain hydrogels undergo volume-phase transition with a change in the temperature of the environmental conditions. The reversible volume change at the transition depends on the degree of ionization and the components of the polymer chains. There is usually a negligible or small positive enthalpy of mixing which opposes the process. However, there is also a large gain in the entropy which drives the process. This type of behavior is related to polymer phase separation as the temperature is raised to a critical value known as the lower critical miscibility or solution temperature (LCST). Networks showing lower critical miscibility temperature tend to shrink or collapse as the temperature is increased above the LCST, and these gels to swell upon lowering the temperature below the LCST.
Temperature sensitive hydrogels are classified as either positive or negative temperature-sensitive systems, depending on whether they are contracted below or above

122 |
NICHOLAS A. PEPPAS AND J. ZACHARY HILT |
a critical temperature, respectively. The majority of the research on thermosensitive hydrogels has focused on poly(N-isopropyl acrylamide) (PNIPAAm), which is a negative temperature-sensitive hydrogel exhibiting a phase transition around 33◦C. PNIPAAm and other thermosensitive hydrogels have been studied for variety of applications, including in drug delivery and tissue engineering [26, 29].
6.1.4. pH-Responsive Hydrogels
In networks containing weakly acidic or basic pendent groups, water sorption can result in ionization of these pendent groups depending on the solution pH and ionic composition. The gels then act as semi-permeable membranes to the counterions influencing the osmotic balance between the hydrogel and the external solution through ion exchange, depending on ion-ion interactions. For ionic gels containing weakly acidic pendent groups, the equilibrium degree of swelling increases as the pH of the external solution increases, while the degree of swelling increases as the pH decreases for gels containing weakly basic pendent groups.
Numerous properties contribute to the swelling of ionic hydrogels. Peppas and Khare [23] discussed the effect of these properties including the ionic content, ionization equilibrium considerations, nature of counterions, and nature of the polymer. An increase in the ionic content of the gel increases the hydrophilicity leading to faster swelling and a higher equilibrium degree of swelling. Anionic networks contain acidic pendant groups, such as carboxylic acid, with a characteristic pKa, while cationic networks contain basic pendant groups, such as amine groups, with a characteristic pKb. In the case of anionic networks, ionization of these acid groups will occur once the pH of the environment is above the characteristic pKa of the acid group, leading to the absorption of water into the polymer to a greater degree causing swelling. This actuation process is shown in Figure 6.3. In an ampholyte, which contains both acidic and basic groups, the isoelectric pH determines the transitional pH of swelling of the gel.
|
|
|
|
|
|
CH3 |
|
CH3 |
|
|
CH3 |
|
CH3 |
|
PMAA |
C CH2 |
|
C CH2 |
‚ |
|
|
|
Chain #1 |
n |
|||||
|
|
|
|
|
|||||
|
|
|
|
|
|
C=O |
C=O |
n |
|
PMAA |
C CH2 |
|
C CH2 |
n‚ |
|
|
|
||
Chain #1 |
n |
|
O- |
|
O- |
|
|||
C=O |
C=O |
|
|
|
|||||
|
|
|
|
|
|
repulsion Ionic |
|
||
|
OH |
|
OH |
|
|
|
Crosslink |
|
|
|
OH |
Crosslink |
OH |
|
|
O- |
|
||
|
|
|
|
|
|
||||
|
C=O |
|
C=O |
|
|
|
O- |
|
|
|
|
|
|
C=O |
|
C=O |
|
||
PMAA |
C CH2 |
‚‚ |
C CH2 |
‚‚‚ |
|
|
|
||
Chain #2 |
PMAA |
|
|
|
|
||||
|
|
|
|
|
|
||||
|
CH3 |
n |
CH3 |
n |
Chain #2 |
C CH2 |
‚‚ |
C CH2 |
‚‚ |
|
|
|
|
|
|
CH3 |
n |
CH3 |
n |
|
|
|
|
|
|
|
|
pH < pKa of the carboxylic acid group |
pH > pKa of the carboxylic acid group |
FIGURE 6.3. Schematic of the pH dependent swelling process of an anionic hydrogel: specifically, a crosslinked poly(methacrylic acid) (PMAA) is illustrated.
INTELLIGENT POLYMERIC NETWORKS IN BIOMOLECULAR SENSING |
123 |
Anionic
Q
Cationic
pH
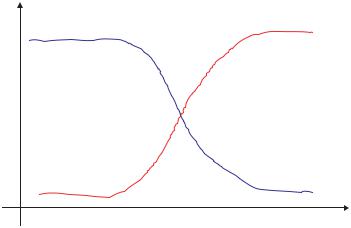
FIGURE 6.4. Equilibrium volume swelling, Q, of ionic hydrogels as a function of pH.
Ionization equilibrium considerations also affect the swelling behavior of ionic hydrogels. Fixed charges in the network lead to the formation of an electric double layer of fixed charges and counterions in the gel. Due to Donnan equilibrium, the chemical potential of the ions inside the gel is equal to that of the ions outside the gel in the swelling medium. Donnan exclusion prevents the sorption of co-ions because of electroneutrality resulting in a higher concentration of counterions in the gel phase than in the external swelling agent. The efficiency of co-ion exclusion, or an increase in the Donnan potential, increases with decreasing solution concentration. Increasing ionic content of the gel also increases the efficiency of co-ion exclusion [30]. A schematic of the characteristic pH response curves for ionic hydrogels is included as Figure 6.4. Examples of some commonly studied ionic polymers include poly(acrylic acid), poly(methacrylic acid), polyacrylamide, poly(diethylaminoethyl methacrylate), and poly(dimethylaminoethyl methacrylate).
6.1.5. Biohybrid Hydrogels
By incorporating biological elements into hydrogel systems, researchers have created hydrogels that are sensitive to specific analytes. For instance, research groups have immobilized enzymes within the network structure of the hydrogel. In the presence of specific chemicals, the enzyme triggers a reaction which changes the microenvironment of the hydrogel. Changes in the local microenvironment (such as pH or temperature) lead to gel swelling or collapse. These systems are completely reversible in nature.
One method is to immobilize into pH-sensitive hydrogel networks enzymes that act on a specific analyte leading to by-products that affect the environmental pH. An example from our studies [31] was the inclusion of activated glucose oxidase into pH-sensitive cationic hydrogels (see Figure 6.5). The glucose oxidase converts glucose into gluconic acid lowering the pH of the local environment, which then causes the hydrogel network to swell in the case of a cationic gel. This system was proposed for a responsive drug delivery system that
124 |
NICHOLAS A. PEPPAS AND J. ZACHARY HILT |
IGOx
GOx I |
I |
|
|
I |
|
I |
G |
GlucA |
GOx |
|
|
|
G |
|
G |
G I |
|
GOx |
GlucA |
|
|
|
|
|
|
|
I |
|
|
Empty hydrogel absorbs glucose leading to gluconic acid production
I |
G |
GlucA GOx |
I |
|
G |
||||
|
||||
|
GOx |
G |
GGlucA |
|
|
G |
|||
|
|
GlucA |
|
Decrease in pH leads to gel expansion which releases insulin
FIGURE 6.5. Actuation mechanism of biohybrid cationic hydrogels with illustration as delivery device. (A) the gel is a crosslinked network with glucose oxidase immobilized in it and insulin is physically entrapped into the system, (B) in the presence of glucose gluconic acid is produced, (C) increase in mesh size results in release of insulin.
would swell and release insulin in response to an increase in glucose concentration, but this biohybrid system has equal merit as a sensing element.
Another approach to impart analyte specificity, based on competitive binding, is loading a hydrogel, having the desired analyte as pendant groups on its chain, with a corresponding entity that selectively binds the analyte. The entity will bind the pendent analytes and form reversible crosslinks in the network, which will then be broken with the competitive binding that takes place in the presence of the analyte. Park et al. [32] immobilized concanavalin A (Con A), a lectin (carbohydrate-binding proteins), in a hydrogel that contained pendant glucose in its network. The Con A non-covalently bound to the glucose pendent groups to form crosslinks, and with increasing concentration of glucose in the environment, the crosslinks were reversibly broken allowing the network to swell.
6.1.6. Biomolecular Imprinted Polymers
Although the above mentioned techniques have been proven effective in producing analyte sensitive hydrogels, they rely on proteins, which inherently lead to limitations in the system. The major limitations of natural receptors, such as proteins, are their high cost, possible antigenicity, and low stability. An alternative to these techniques is to use synthetic biomimetic methods to create hydrogels that will bind and respond to specific analytes. These biomimetic polymer networks are advantageous because they can be tailored to bind any molecule with controlled selectivity and affinity, provided that certain interactions exist. In a recent review, several methods utilizing molecular imprinting processes, a template mediated polymerization, were proposed to design analyte responsive hydrogels that can
