
Biomolecular Sensing Processing and Analysis - Rashid Bashir and Steve Wereley
.pdfAN ON-CHIP ARTIFICIAL PORE FOR MOLECULAR SENSING |
53 |
[9]E.C. Gregg and K. David Steidley. Electrical counting and sizing of mammalian cells in suspension. Biophys. J., 5:393–405, 1965.
[10]R.W. DeBlois and C.P. Bean. Counting and sizing of submicron particles by the resistive pulse technique. Rev. Sci. Inst., 41:909–913, 1970.
[11]M. Koch, A.G.R. Evans, and A. Brunnschweiler. Design and fabrication of a micromachined coulter counter.
J.Micromech. Microeng., 9:159–161, 1999.
[12]O.A. Saleh and L.L. Sohn. Quantitative sensing of nanoscale colloids using a microchip Coulter counter. Rev. Sci. Inst., 72:4449–4451, 2001.
[13]D.W. Fakes, M.C. Davies, A. Browns, and J.M. Newton. The surface analysis of a plasma modified contactlens surface by SSIMS. Surf. Interface Anal., 13:233–236, 1988.
[14]M.K. Chaudhury and G.M. Whitesides. Direct measurement of interfacial interactions between semispherical lenses and flat sheets of poly(dimethylsiloxane) and their chemical derivatives. Langmuir, 7:1013–1025, 1991.
[15]Y.N. Xia and G.M. Whitesides. Angewandte Chemie-International Edition, 37:551–575, 1998.
[16]O.A. Saleh and L.L. Sohn. Correcting off-axis effects on an on-chip resistive-pulse analyzer. Rev. Sci. Inst., 73:4396–4398, 2002.
[17]L.I. Berge, T. Jossang, and J. Feder. Off-axis response for particles passing through long aperatures in Coulter-type counters. Measure. Sci. Technol., 1(6):471, 1990.
[18]H.L. Goldsmith and S.G. Mason. Flow of suspensions through tubes .1. Single spheres, rods, and discs. J. Colloid Sci., 17:448, 1962.
[19]P.B. Luppa, L.J. Sokoll, and D.W. Chan. Immunosensors–principles and applications to clinical chemistry. Clin. Chim. Acta, 314:1–26, 2001.
[20]T. Vo-Dinh and B. Cullum. Biosensors and biochips: Advances in biological and medical diagnostics. Fresen.
J.Anal. Chem., 366:540–551, 2000.
[21]A.P. Turner. Biochemistry: Biosensors–sense and sensitivity. Science, 290:1315–1317, 2000.
[22]R.I. Stefan, J.F. van Staden, and H.Y. Aboul-Enein. Immunosensors in clinical analysis. Fresen. J. Anal. Chem., 366:659–668, 2000.
[23]T.T. Ngo. Developments in immunoassay technology. Methods, 22:1–3, 2000.
[24]O.A. Saleh and L.L. Sohn. Direct detection of antibody-antigen binding using an on-chip artificial pore. Proc. Natl. Acad. Sci., 100:820–824, 2003.
[25]L.Q. Gu, S. Cheley, and H. Bayley. Capture of a single molecule in a nanocavity. Science, 291:636–640, 2001.
[26]Y.K. Sykulev, D.A. Sherman, R.J. Cohen, and H.N. Eisen. Quantitation of reversible binding by particle counting: Hapten-antibody interaction as a model system. Proc. Natl. Acad. Sci. U.S.A., 89:4703–4707, 1992.
[27]G.K. von Schulthess, G.B. Benedek, and R.W. Deblois. Experimental measurements of the temporal evolution of cluster size distributions for high-functionality antigens cross-linked by antibody. Macromolecules, 16:434–440, 1983.
[28]G.K. von Schulthess, G.B. Benedek, and R.W. Deblois. Measurement of the cluster size distributions for high functionality antigens cross-linked by antibody. Macromolecules, 13:939–945, 1980.
[29]G.K. von Schulthess, R.W. Deblois, and G.B. Benedek. Agglutination of antigen coated carrier particles by antibody. Biophys. J., 21:A115–A115, 1978.
[30]W.M. Mullett, E.P. Lai, and J.M. Yeung. Surface plasmon resonance-based immunoassays. Methods, 22:77– 91, 2000.
[31]G.M. Whitesides and A.D. Stroock. Flexible methods for microfluidics. Phys. Today, 54:42–48, 2001.
[32]T. Chovan and A. Guttman. Microfabricated devices in biotechnology and biochemical processing. Trends Biotechnol, 20:116–122, 2002.
[33]W.R. Smythe. Off-axis particles in coulter type counters. Rev. Sci. Inst., 43:817, 1972.
[34]M.M. Bradford. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal. Biochem., 72:248–254, 1976.
[35]M. Bruchez, Jr., M. Moronne, P. Gin, S. Weiss, and A.P. Alivisatos. Semiconductor nanocrystals as fluorescent biological labels. Science, 281:2013–2016, 1998.
[36]A. Carbonaro and L.L.A Sohn. resistive-pulse sensor chip for multianalyte immunoassays. Lab on a Chip, 5:1155–1160, 2005.
[37]O.A. Saleh and L.L. Sohn. An artificial nanopore for molecular sensing. NanoLetters, 3:37–38, 2003.
[38]H. Schmid and B. Michel. Siloxane polymers for high-resolution, high-accuracy soft lithography. Macromolecules, 33(8):3042–3049, 2000.
4
Cell Based Sensing Technologies
Cengiz S. Ozkan, Mihri Ozkan, Mo Yang, Xuan Zhang,
Shalini Prasad, and Andre Morgan
Mechanical Engineering Department, University of California, Riverside, CA 92521, USA
Biosensor technology is the driving force in the development of biochips capable of detecting and analyzing biomolecules. A biosensor is a device that detects, records, and transmits information regarding a physiological change or the presence of various chemical or biological materials in the environment. Cell based sensing is the most promising alternative to the existing bio-sensing techniques as cells have the capability of identifying very minute concentrations of environmental agents. The use of living cells as sensing elements provides the opportunity for high sensitivity to a broad range of chemically active substances which affect the electrochemical activity of cells. This chapter provides an overview of the development of cell-based sensors for biological and chemical detection applications, along with significant advances over the last several years. Special emphasis will be given on recently developed planar microelectrode arrays for enabling extracellular recording from electrochemically active cells cultured in vivo. The extracellular signal spectrum can be modulated when the cells are exposed to a variety of chemical agents and this modulated signal constitutes a “signature pattern” which serves as the finger print for a specific chemical agent. Cell based sensors can change the sensing paradigm from “detect-to-treat” to “detect-to-warn”.
4.1. OVERVIEW
General interest in biosensors has grown considerably since the description by Updike and Hicks [94] of the first functional enzyme electrode based on glucose oxidase deposited on an oxygen sensor. The last decade in particular has seen efforts within both academic and commercial sectors being directed towards the development of practical biosensors.
56 |
CENGIZ S. OZKAN ET AL. |
However, it is important to realize that advances in allied subject areas have been important in aiding these research activities. The field of biotechnology has contributed enormously by providing an increased understanding of immobilized bioreagents and improved techniques for immobilization, and purely technological advances in the microelectronics and communication industries have provided more refined transducer elements and devices. Today, biosensor technology is the driving force in the development of biochips for the detection of gaseous pollutants [100], biological and chemical pollutants [43], pesticides [74], and micro-organisms [85]. Biosensors combine the selectivity of biology with the processing power of modern microelectronics and optoelectronics to offer powerful new analytical tools with major applications in medicine, environmental diagnostics and the food and processing industries. A novel challenge is the development of effective and multifunctional biosensors based on fundamental research in biotechnology, genetics and information technology, such that the existing axiom of “detect -to-treat” would change to “detect -to-warn”.
Conventional methods for detecting environmental threats are primarily based on enzyme [48], antibody [10, 84], or nucleic acid-based assays [20, 65, 96], which rely on chemical properties or molecular recognition to identify a particular agent [103]. The current method involved in risk assessment for humans, fail in field situations due to their inability to detect large numbers of chemical agents, characterize the functionality of agents and determine the human performance decrements [62].
4.2. CELL-BASED BIOSENSORS
Cell based sensing [81] is the most promising alternative to the existing bio-sensing techniques as cells have the capability of identifying very minute concentrations of environmental agents. In cell based sensing, mammalian cells with excitable cell membranes are used as biosensors. The membranes of mammalian are comprised of ion channels, which open or close based on the changes in the internal and external local environment of the cells. This results in the development of ionic current gradients that are responsible for the modification of the electrical conductivity. Cells express and sustain an array of potential molecular sensors. Receptors, channels and enzymes that are sensitive to an analyte are maintained in a physiologically relevant manner native to the cellular machinery. In contrast with the antibody-based approaches, cell-based sensors should optimally only respond to functional biologically active analytes. It is also important to note that there are two major difficulties involved in using cells as sensors: it requires the knowledge of microbiology or tissue culture, and the lifetime of living cells is usually more limited than enzymes [51]. Nevertheless, there are a number of compelling motivations that make it very attractive to work with living cells for sensing applications. The first and most important one is that only by using a living component it is possible to obtain functional information, i.e. information about the effect of a stimulus on a living system. This can be contrasted with analytical information, which answers the question of how much of a given substance is present. There are many circumstances in which the type of information required is really functional, and analytical tests are carried out only to estimate the functional consequences of the substances being investigated. In those cases, a measurement method using a living system is very attractive because it can yield that functional information directly, which provides real-time sensing capability.
CELL BASED SENSING TECHNOLOGIES |
57 |
Using a living cell as the sensing element, one can also obtain analytical information, both qualitatively and quantitatively. In its simplest form, it tells us whether a given substance is present, and in what concentration. Cells with a given type of receptors can be considered as sensors for agonists, with a sensitivity determined by the binding constant of that receptor/ligand combination [5, 78]. Another large body of work uses bacteria, often genetically engineered to respond to specific substances. Using amperometric detection, it has been possible to detect herbicides [101], benzene [87], alcohol [44], and trimethylamine gas [50]. Another detection method which has recently become popular for bacteria is bioluminescence. One of the advantages invoked for the use of cells in environmental applications is that it allows the measurement of the total bioavailability of a given pollutant rather than its free form [36, 76]. For instance, a bioluminescent bacteria detector specific for copper also detects insoluble copper sulfide [95]. This means that the analytical question really becomes a functional one, namely how bio-functional a given substance is. When used for environmental applications, a further potential advantage of biosensor devices is that they are capable of continuous monitoring, and can be made small enough to use in the field rather than in the laboratory. The main potential problem is the handling and lifetime of the living component.
Cells express and sustain an array of potential molecular sensors. Receptors, channels, and enzymes that may be sensitive to an analyte are maintained in a physiological relevant manner by a native cellular machinery. In contrast with antibody-based approaches, cell-based biosensors should optimally only respond to functional, biologically active analytes. Cell-based biosensors have been implemented using microorganisms, There are several approaches for transduction of cell signals including cell fluorescence, metabolism, impedance, intracellular potentials and extracellular potentials.
4.2.1. Cellular Microorganism Based Biosensors
Metabolism cell stress can activate microorganism pathways due to some analytes, such as pollutants [3]. The members of bacteria was sensitive to several groups of chemicals including phenols, halomethanes and several oxidants responding by increased luminescence to a different type of environmental stress. Cell biosensor specific for formaldehyde was developed using double-mutant cells of the methylotrophic yeast, where the activities of some of the enzymes in the metabolic pathway of the wild-strain cells were deliberately suppressed by introducing respective genetic blocks to optimize the selectivity and acidification rate [47]. Mutant yeast cells produced in this way were immobilized in Ca-alginate gel on the gate of a pH-sensitive field effect transistor. Another sensor approach is based on genetically engineered bacteria such as a bioluminescent catabolic reporter bacterium developed for continuous on-line monitoring of naphthalene and salicylate bioavailability and microbial catabolic activity potential in waste streams [36]. The bioluminescent reporter bacterium, Pseudomonas fluorescens HK44, carries a transcriptional nahG-luxCDABE fusion for naphthalene and salicylate catabolism. Exposure to either compound resulted in inducible bioluminescence. Engineered bacteria were used as whole cell sensor elements for detecting benzene [2], toluene [7], mercury [76] and octane [83]. The alteration of a microorganism-based biosensor response is important and genetic detection is favored by insufficient selectivity [21]. Cell-based biosensors for genetic detection derived from a biological system of interest can offer functional and physiologically relevant information.
58 |
CENGIZ S. OZKAN ET AL. |
4.2.2. Fluorescence Based Cellular Biosensors
Fluorescence based sensors are showing several signs of wide-ranging development [14, 16] for the clarification of the underlying photophysics, the discovery of several biocompatible systems, and the demonstration of their usefulness in cellular environments. Another sign is that the beneficiaries of the field are multiplying. They range from medical diagnostics through physiological imaging, biochemical investigations, environmental monitoring, and chemical analysis to aeronautical engineering.
The design of fluorescent molecular sensors for chemical species combines a receptor and a fluorophore for a “catch-and-tell” operation. The receptor module engages exclusively in transactions of chemical species. On the other hand, the fluorophore is concerned solely with photon emission and absorption. Molecular light emission is particularly appealing for sensing purposes owing to its near-ultimate detectability, “off/on” switchability, and very high spatiotemporal resolution including video imaging. The commonest approaches to combining fluorophore and receptor modules involve integrated or spaced components [4]. New fluorescence reagents based on the combination of molecular biology, fluorescent probe chemistry and protein chemistry have been developed for cell-based assays. Variants of the green fluorescent protein (GFP) with different colors would be very useful for simultaneous comparisons of multiple protein fates, developmental lineages and gene expression levels [37]. The simplest way to shift the emission color of GFP is to substitute histidine or tryptophan for the tyrosine in the chromophore, but such blue-shifted point mutants are only dimly fluorescent. The longest wavelengths previously reported for the excitation and emission peaks of GFP mutants are 488 and 511 nm, respectively.
The integrated or intrinsic sensor format relies on internal charge transfer within the excited state. The partial electronic charges so separated can interact with the target species when it is trapped by the receptor. The energy of the excited state is thereby disturbed and shows up as a blueor red-shifted light absorption and/or emission [93]. The separation of charges within the spaced or conjugate sensor format occurs after excited state creation. This is photo-induced electron transfer (PET), which competes against fluorescence to dominate the energy dissipation of the excited state, i.e., fluorescence is switched off when the target species is absent. When it arrives, however, PET is arrested and fluorescence regains the upper hand, i.e., fluorescence is switched on. Czarnik’s compound [41], de Silva’s compound [15], and Calcium Green-1 from Molecular Probes [35] respond dramatically to Zn2+, H+, and Ca2+, respectively.
Sensors for cell-based applications developed in this manner reveal that intracellular ionic signals are heterogeneous at the single-cell level [93]. To analyze whether this heterogeneity is preserved in downstream events, a sensitive, single-cell assay for gene expression was developed. The reporting molecule is the bacterial enzyme β-lactamase, which generates an amplified signal by changing the fluorescence of a substrate made available intracellularly.
4.2.3. Impedance Based Cellular Biosensors
The electrical properties of biological material have been studied using suitable instrumentation. Impedance techniques have been used to study organs in the body [17], explanted neural tissues [12, 39], whole blood and erythrocytes [22, 23], cultured cell
CELL BASED SENSING TECHNOLOGIES |
59 |
suspensions [73], bacterial growth monitoring [34], and anchorage dependent cell cultures [26]. There is a great deal of relevant information regarding the characteristics of biological material to be obtained from those studies. Most significant are the frequency dependent dielectric properties of biological materials including cells which yield insight into the expected behavior within different frequency ranges.
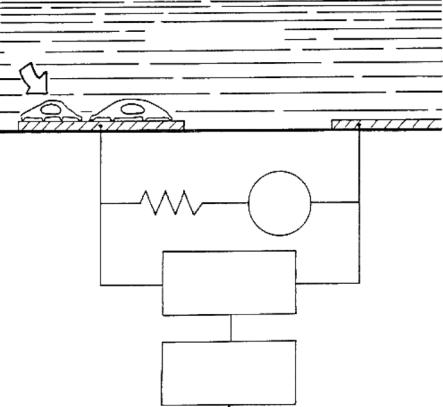
The membranes of biological materials including cells exhibit dielectric properties. By measuring the changes in the effective electrode impedance, cultured cell adhesion, spreading and motility can be interpreted from the extracellular signal of the cells. The reliability of impedance measurements depends on the observation that intact living cells are excellent electrical insulators at low signal frequencies. When the coverage over an electrode area increases, the effective electrode impedance increases as well. Impedance measurements have been used for monitoring the behavior of an array of nonexcitable cell types including macrophages [46], endothelial cells [90] and fibroblasts [26]. Figure 4.1 shows the schematic of an impedance sensor.
It is desirable to improve the interfacial sealing at the cell-electrode junction for conducting the measurement of action potentials extracellularly [86]. Further work has been done to deduce cell-electrode interface characteristics for the development of a better understanding of extracellular action potential measurements (Lind, et al., 1991). Surface roughness effects on cell adhesion were examined by looking at smooth gold electrodes, rough platinized gold electrodes, and gold electrodes roughened by dry etching (Lind, et al., 1991). In 1995, the work was continued using Lymnaea neurons [6]. Impedance measurements were performed both before and during cell culture and estimates of the cell to substrate sealing impedance were made. These impedance values were then correlated with the recorded extracellular action potentials, revealing a directly proportional relationship. As the sealing impedance increased, the extracellular signal strength did as well, thereby verifying that the sealing impedance is indeed critical for improved signal to noise ratio (SNR).
4.2.4. Intracellular Potential Based Biosensors
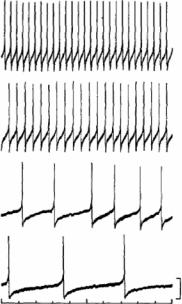
The functional or physiological significance of the analyte to the organism can be related to the information derived from cell-based biosensors. Bioelectric signals from excitable cells have been used to relay functional information concerning cell status [28]. Membrane excitability plays a key physiological role in primary cells for the control of secretion and contraction, respectively. Thus, analytes that affect membrane excitability in excitable cells are expected to have profound effects on an organism. Furthermore, the nature of the changes in excitability can yield physiological implications for the response of the organism to analytes. Direct monitoring of cell membrane potential can be achieved through the use of glass microelectrodes. Repetitively firing neurons from the visceral ganglia of the pond snail has been used to quantitatively assess the concentration of a model analyte, serotonin [80]. Figure 4.2 portrays an example of the graded increase in firing rate seen in both the VV1 and VV2 neurons with serotonin concentration. As indicated in the figure, the traces are the different cellular response to additions of 10−6 M, 10−5 M, 10−4 M, and 10−3 M serotonin. The basic principle behind intracellular measurements is that tissue slices are prepared and are exposed to chemical analytes under test and the electrical activity from excitable cells are measured using the patch clamp technique. This
60 |
CENGIZ S. OZKAN ET AL. |
TISSUE CULTURE MEDIUM (ELECTROLYTE)
CELLS
SMALL GOLD |
LARGE GOLD |
ELECTRODE |
COUNTER ELECTRODE |
(10-4CM2) |
(101CM2) |
4000 Hz
AC SIGNAL
1 VOLT
1 MΩ
LOCK-IN
AMPLIFIER
PC
DATA ACQUISITION
AND PROCESSING
FIGURE 4.1. Schematic of an impedance sensor. Impedance of the small electrode is measured with a lock-in amplifier in series with a 1M resistor to obtain an approximate constant current source. Electric cell–substrate impedance sensing (ECIS) is the technique that is used to monitor attachment and spreading of mammalian cells quantitatively and in real time. The method is based on measuring changes in AC impedance of small gold-film electrodes deposited on a culture dish and used as growth substrate. The gold electrodes are immersed in the tissue culture medium. When cells attach and spread on the electrode, the measured electrical impedance changes because the cells constrain the current flow. This changing impedance is interpreted to reveal relevant information about cell behaviors, such as spreading, locomotion and motility. They involve the coordination of many biochemical events [108].
technique illustrates the utility of excitable cells as sensors with sensitivity to chemical warfare agents; however, the invasive nature of intracellular recording significantly limits the robustness of this approach for biosensor applications. Another drawback is that excitable cells assemble into coupled networks rather than acting as isolated elements; as a result, for certain sensing applications the ability to simultaneously monitor two or more cells is essential as it permits measurements of membrane excitability and cell coupling. This is not possible using intracellular techniques. The advantage of the technique is that the physiological state of a cell can be assessed. Due to the invasiveness of the technique, it is not possible to apply it for long term measurements.
CELL BASED SENSING TECHNOLOGIES |
61 |
Evaluation of neuron-based sensing with serotonin
10−3M
10−4M
10−5M
10−6M |
20 mV |
|
|
|
10 sec |
FIGURE 4.2. An example of the effects of serotonin on the spontaneous firing rate of the VV1 and VV2 neurons in a Limnea stagnalis snail. As indicated, the traces are the cellular response to additions of 10−6 M, 10−5 M, 10−4 M, and 10−3 M serotonin [80].
4.2.5. Extracellular Potential Based Biosensors
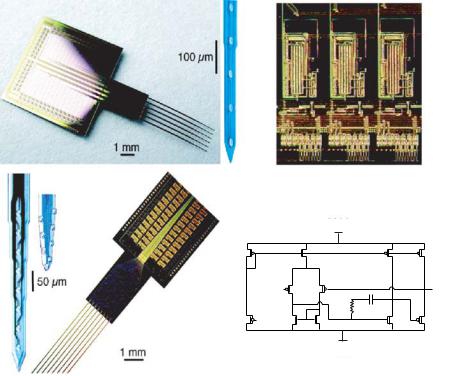
In recent years, the use of microfabricated extracellular electrodes to monitor the electrical activity in cells has been used more frequently. Extracellular microelectrode arrays offer a noninvasive and long-term approach to the measurement of bio-potentials [11]. Multi-electrode arrays, typically consisting of 16 to 64 recording sites, present a tremendous conduit for data acquisition from networks of electrically active cells. The invasive nature of intracellular recording, as well as voltage-sensitive dyes, limits the utility of standard electrophysiological measurements and optical approaches. As a result, planar microelectrode arrays have emerged as a powerful tool for long term recording of network dynamics. Extracellular recordings have been achieved from dissociated cells as well; that is more useful in specific chemical agent sensing applications. The current state of the art microelectrode technology comprises of 96 microelectrodes fabricated using standard lithography techniques as shown in Figure 4.3A [13]. More detailed work by Gross and his colleagues at the University of North Texas over the past 20 yrs have demonstrated the feasibility of neuronal networks for biosensor applications [28, 29]. They have utilized transparent patterns of indium–tin–oxide conductors 10 µm wide, which were photo-etched and passivated with a polysiloxane resin [30, 31]. Laser de-insulation of the resin resulted in 64 recording “craters” over an area of 1 mm2, suitable for sampling of the neuronal ensembles achieved in culture.
62 |
|
CENGIZ S. OZKAN ET AL. |
|
Principle of extra cellular potential based biosensors |
|
(A) |
A |
C |
B1 |
B |
D |
|
|
|
|
B2 |
|
|
Vdd |
|
|
|
|
|
|
|
|
|
W/L=36/3 |
|
|
W/L=80/3 |
|
|
|
|
W/L=36/3 |
W/L=20/3 |
|
|
|
W/L=600/9 |
W/L=600/9 |
|
|
|
|
Vin |
|
Vout |
|
|
|
|
|
|
|
|
|
|
36 kΩ |
20pF |
|
|
W/L=36/3 |
W/L=36/9 |
W/L=36/9 |
W/L=231/3 |
|
|
|
|
W/L=12/3 |
|
|
|
|
|
|
|
Vss
FIGURE 4.3. (A) Extra cellular multiple-site recording probes. A: 6-shank, 96-site passive probe for 2-dimensional imaging of field activity. Recording sites (16 each; 100 µm vertical spacing) are shown at higher magnification. B: 8-shank, 64-site active probe. Two different recording site configurations (linear, B1 and staggered sites, B2) are shown as insets. C: close-up of on-chip buffering circuitry. Three of the 64 amplifiers and associated circuits are shown. D: circuit schematic of operational amplifier for buffering neural signals) [13].
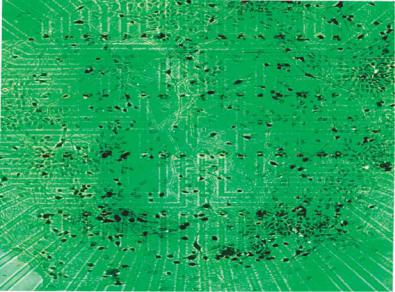
Indeed, neurons cultured over microelectrode arrays have shown regular electrophysiological behavior and stable pharmacological sensitivity for over 9 months [32]. Figure 4.3B shows neuronal cultures obtained on a microelectrode array with 64 sites [109]. In fact, their precise methodological approach generates a co-culture of glial support cells and randomly seeded neurons, resulting in spontaneous bioelectrical activity ranging from stochastic neuronal spiking to organized bursting and long-term oscillatory activity [32]. Microelectrode arrays coupled with “turnkey” systems for signal processing and data acquisition are now commercially available. In spite of the obvious advantages of the microelectrode array technology for biosensing, in determining the effect of chemical analytes at the single cell level, it becomes essential to pattern the dissociated cells accurately over the microelectrodes. Single cell based sensing forms the basis for determining cellular sensitivity to a wide range of chemical analytes and determining the cellular physiological changes. Analysis of the extracellular electrical activity provides unique identification tags associated with cellular response to each specific chemical agent also known as “Signature Patterns”.
CELL BASED SENSING TECHNOLOGIES |
63 |
(B)
FIGURE 4.3. (Continued ) (B) Neuronal cultures on a 64 microelectrode array. Laser de-insulation of the resin resulted in 64 recording “craters” over an area of 1 mm2, suitable for sampling the neuronal ensembles achieved in culture. neurons cultured over microelectrode arrays have shown regular electrophysiological behavior and stable pharmacological sensitivity for over 9 months [109].
4.3. DESIGN AND METHODS
4.3.1. Requirements for Cell Based Sensors
When developing a system for monitoring the extracellular action potential or cellular impedance of anchorage dependent cell types, it is necessary to design the sensing system with several criteria in mind: Biocompatibility, maintenance of the physiochemical environment (temperature, pH, etc.), maintenance of sterility during cell growth and sample introduction, methods of sample introduction, a transducer for monitoring the desired electrical signal, low signal path parasitics, electronics for extraction of the electrical signal, and packaging which facilitates insertion of the cell culture system in to the measurement electronics while protecting the living system from the external environment. These requirements often trade off against each other and require compromise for the best overall solution. Biocompatibility is perhaps the most important consideration when developing a cell based biosensor. If biocompatible materials are not employed through the design, the sensing element (the cells) will not survive to perform the initial signal transduction required. While biocompatibility generally means not having a toxic, harmful, or otherwise deleterious effect on biological function, there are varying degrees of it dependent on the application. Chronic studies where foreign materials are in contact with living tissue require a more diligent effort for the determination of biocompatibility than do acute studies where the tissue is in contact with the materials for a short duration. Cell culture for hybrid biosensor applications falls somewhere in between, depending on the application area. For all of
