
- •Contents
- •Contributors
- •Part I General Principles of Cell Death
- •1 Human Caspases – Apoptosis and Inflammation Signaling Proteases
- •1.1. Apoptosis and limited proteolysis
- •1.2. Caspase evolution
- •2. ACTIVATION MECHANISMS
- •2.2. The activation platforms
- •2.4. Proteolytic maturation
- •3. CASPASE SUBSTRATES
- •4. REGULATION BY NATURAL INHIBITORS
- •REFERENCES
- •2 Inhibitor of Apoptosis Proteins
- •2. CELLULAR FUNCTIONS AND PHENOTYPES OF IAP
- •3. IN VIVO FUNCTIONS OF IAP FAMILY PROTEINS
- •4. SUBCELLULAR LOCATIONS OF IAP
- •8. IAP–IAP INTERACTIONS
- •10. ENDOGENOUS ANTAGONISTS OF IAP
- •11. IAPs AND DISEASE
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2.1. The CD95 (Fas/APO-1) system
- •2.1.1. CD95 and CD95L: discovery of the first direct apoptosis-inducing receptor-ligand system
- •2.1.2. Biochemistry of CD95 apoptosis signaling
- •2.2. The TRAIL (Apo2L) system
- •3.1. The TNF system
- •3.1.1. Biochemistry of TNF signal transduction
- •3.1.2. TNF and TNF blockers in the clinic
- •3.2. The DR3 system
- •4. THE DR6 SYSTEM
- •6. CONCLUDING REMARKS AND OUTLOOK
- •SUGGESTED READINGS
- •4 Mitochondria and Cell Death
- •1. INTRODUCTION
- •2. MITOCHONDRIAL PHYSIOLOGY
- •3. THE MITOCHONDRIAL PATHWAY OF APOPTOSIS
- •9. CONCLUSIONS
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •3. INHIBITING APOPTOSIS
- •4. INHIBITING THE INHIBITORS
- •6. THE BCL-2 FAMILY AND CANCER
- •SUGGESTED READINGS
- •6 Endoplasmic Reticulum Stress Response in Cell Death and Cell Survival
- •1. INTRODUCTION
- •2. THE ESR IN YEAST
- •3. THE ESR IN MAMMALS
- •4. THE ESR AND CELL DEATH
- •5. THE ESR IN DEVELOPMENT AND TISSUE HOMEOSTASIS
- •6. THE ESR IN HUMAN DISEASE
- •7. CONCLUSION
- •7 Autophagy – The Liaison between the Lysosomal System and Cell Death
- •1. INTRODUCTION
- •2. AUTOPHAGY
- •2.2. Physiologic functions of autophagy
- •2.3. Autophagy and human pathology
- •3. AUTOPHAGY AND CELL DEATH
- •3.1. Autophagy as anti–cell death mechanism
- •3.2. Autophagy as a cell death mechanism
- •3.3. Molecular players of the autophagy–cell death cross-talk
- •4. AUTOPHAGY, CELLULAR DEATH, AND CANCER
- •5. CONCLUDING REMARKS AND PENDING QUESTIONS
- •SUGGESTED READINGS
- •8 Cell Death in Response to Genotoxic Stress and DNA Damage
- •1. TYPES OF DNA DAMAGE AND REPAIR SYSTEMS
- •2. DNA DAMAGE RESPONSE
- •2.2. Transducers
- •2.3. Effectors
- •4. CHROMATIN MODIFICATIONS
- •5. CELL CYCLE CHECKPOINT REGULATION
- •6. WHEN REPAIR FAILS: SENESCENCE VERSUS APOPTOSIS
- •6.1. DNA damage response and the induction of apoptosis
- •6.2. p53-independent mechanisms of apoptosis
- •6.3. DNA damage response and senescence induction
- •7. DNA DAMAGE FROM OXIDATIVE STRESS
- •SUGGESTED READINGS
- •9 Ceramide and Lipid Mediators in Apoptosis
- •1. INTRODUCTION
- •3.1. Basic cell signaling often involves small molecules
- •3.2. Sphingolipids are cell-signaling molecules
- •3.2.1. Ceramide induces apoptosis
- •3.2.2. Ceramide accumulates during programmed cell death
- •3.2.3. Inhibition of ceramide production alters cell death signaling
- •4.1. Ceramide is generated through SM hydrolysis
- •4.3. aSMase can be activated independently of extracellular receptors to regulate apoptosis
- •4.4. Controversial aspects of the role of aSMase in apoptosis
- •4.5. De novo ceramide synthesis regulates programmed cell death
- •4.6. p53 and Bcl-2–like proteins are connected to de novo ceramide synthesis
- •4.7. The role and regulation of de novo synthesis in ceramide-mediated cell death is poorly understood
- •5. CONCLUDING REMARKS AND FUTURE DIRECTIONS
- •5.1. Who? (Which enzyme?)
- •5.2. What? (Which ceramide?)
- •5.3. Where? (Which compartment?)
- •5.4. When? (At what steps?)
- •5.5. How? (Through what mechanisms?)
- •5.6. What purpose?
- •6. SUMMARY
- •SUGGESTED READINGS
- •1. General Introduction
- •1.1. Cytotoxic lymphocytes and apoptosis
- •2. CYTOTOXIC GRANULES AND GRANULE EXOCYTOSIS
- •2.1. Synthesis and loading of the cytotoxic granule proteins into the secretory granules
- •2.2. The immunological synapse
- •2.3. Secretion of granule proteins
- •2.4. Uptake of proapoptotic proteins into the target cell
- •2.5. Activation of death pathways by granzymes
- •3. GRANULE-BOUND CYTOTOXIC PROTEINS
- •3.1. Perforin
- •3.2. Granulysin
- •3.3. Granzymes
- •3.3.1. GrB-mediated apoptosis
- •3.3.2. GrA-mediated cell death
- •3.3.3. Orphan granzyme-mediated cell death
- •5. CONCLUSIONS
- •REFERENCES
- •Part II Cell Death in Tissues and Organs
- •1.1. Death by trophic factor deprivation
- •1.2. Key molecules regulating neuronal apoptosis during development
- •1.2.1. Roles of caspases and Apaf-1 in neuronal cell death
- •1.2.2. Role of Bcl-2 family members in neuronal cell death
- •1.3. Signal transduction from neurotrophins and neurotrophin receptors
- •1.3.1. Signals for survival
- •1.3.2. Signals for death
- •2.1. Apoptosis in neurodegenerative diseases
- •2.1.4. Amyotrophic lateral sclerosis
- •2.2. Necrotic cell death in neurodegenerative diseases
- •2.2.1. Calpains
- •2.2.2. Cathepsins
- •3. CONCLUSIONS
- •ACKNOWLEDGMENT
- •SUGGESTED READINGS
- •ACKNOWLEDGMENT
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •5. S-NITROSYLATION OF PARKIN
- •7. POTENTIAL TREATMENT OF EXCESSIVE NMDA-INDUCED Ca2+ INFLUX AND FREE RADICAL GENERATION
- •8. FUTURE THERAPEUTICS: NITROMEMANTINES
- •9. CONCLUSIONS
- •Acknowledgments
- •SUGGESTED READINGS
- •3. MITOCHONDRIAL PERMEABILITY TRANSITION ACTIVATED BY Ca2+ AND OXIDATIVE STRESS
- •4.1. Mitochondrial apoptotic pathways
- •4.2. Bcl-2 family proteins
- •4.3. Caspase-dependent apoptosis
- •4.4. Caspase-independent apoptosis
- •4.5. Calpains in ischemic neural cell death
- •5. SUMMARY
- •ACKNOWLEDGMENTS
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2. HISTORICAL ANTECEDENTS
- •7.1. Activation of p21 waf1/cip1: Targeting extrinsic and intrinsic pathways to death
- •8. CONCLUSION
- •ACKNOWLEDGMENTS
- •REFERENCES
- •16 Apoptosis and Homeostasis in the Eye
- •1.1. Lens
- •1.2. Retina
- •2. ROLE OF APOPTOSIS IN DISEASES OF THE EYE
- •2.1. Glaucoma
- •2.2. Age-related macular degeneration
- •4. APOPTOSIS AND OCULAR IMMUNE PRIVILEGE
- •5. CONCLUSIONS
- •SUGGESTED READINGS
- •17 Cell Death in the Inner Ear
- •3. THE COCHLEA IS THE HEARING ORGAN
- •3.1. Ototoxic hair cell death
- •3.2. Aminoglycoside-induced hair cell death
- •3.3. Cisplatin-induced hair cell death
- •3.4. Therapeutic strategies to prevent hair cell death
- •3.5. Challenges to studies of hair cell death
- •4. SPIRAL GANGLION NEURON DEATH
- •4.1. Neurotrophic support from sensory hair cells and supporting cells
- •4.2. Afferent activity from hair cells
- •4.3. Molecular manifestations of spiral ganglion neuron death
- •4.4. Therapeutic interventions to prevent SGN death
- •ACKNOWLEDGMENTS
- •SUGGESTED READINGS
- •18 Cell Death in the Olfactory System
- •1. Introduction
- •2. Anatomical Aspects
- •3. Life and Death in the Olfactory System
- •3.1. Olfactory epithelium
- •3.2. Olfactory bulb
- •REFERENCES
- •1. Introduction
- •3.1. Beta cell death in the development of T1D
- •3.2. Mechanisms of beta cell death in type 1 diabetes
- •3.2.1. Apoptosis signaling pathways downstream of death receptors and inflammatory cytokines
- •3.2.2. Oxidative stress
- •3.3. Mechanisms of beta cell death in type 2 diabetes
- •3.3.1. Glucolipitoxicity
- •3.3.2. Endoplasmic reticulum stress
- •5. SUMMARY
- •Acknowledgments
- •REFERENCES
- •20 Apoptosis in the Physiology and Diseases of the Respiratory Tract
- •1. APOPTOSIS IN LUNG DEVELOPMENT
- •2. APOPTOSIS IN LUNG PATHOPHYSIOLOGY
- •2.1. Apoptosis in pulmonary inflammation
- •2.2. Apoptosis in acute lung injury
- •2.3. Apoptosis in chronic obstructive pulmonary disease
- •2.4. Apoptosis in interstitial lung diseases
- •2.5. Apoptosis in pulmonary arterial hypertension
- •2.6. Apoptosis in lung cancer
- •SUGGESTED READINGS
- •21 Regulation of Cell Death in the Gastrointestinal Tract
- •1. INTRODUCTION
- •2. ESOPHAGUS
- •3. STOMACH
- •4. SMALL AND LARGE INTESTINE
- •5. LIVER
- •6. PANCREAS
- •7. SUMMARY AND CONCLUDING REMARKS
- •SUGGESTED READINGS
- •22 Apoptosis in the Kidney
- •1. NORMAL KIDNEY STRUCTURE AND FUNCTION
- •3. APOPTOSIS IN ADULT KIDNEY DISEASE
- •4. REGULATION OF APOPTOSIS IN KIDNEY CELLS
- •4.1. Survival factors
- •4.2. Lethal factors
- •4.2.1. TNF superfamily cytokines
- •4.2.2. Other cytokines
- •4.2.3. Glucose
- •4.2.4. Drugs and xenobiotics
- •4.2.5. Ischemia-reperfusion and sepsis
- •5. THERAPEUTIC APPROACHES
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2. APOPTOSIS IN THE NORMAL BREAST
- •2.1. Occurrence and role of apoptosis in the developing breast
- •2.2.2. Death ligands and death receptor pathway
- •2.2.4. LIF-STAT3 proapoptotic signaling
- •2.2.5. IGF survival signaling
- •2.2.6. Regulation by adhesion
- •2.2.7. PI3K/AKT pathway: molecular hub for survival signals
- •2.2.8. Downstream regulators of apoptosis: the BCL-2 family members
- •3. APOPTOSIS IN BREAST CANCER
- •3.1. Apoptosis in breast tumorigenesis and cancer progression
- •3.2. Molecular dysregulation of apoptosis in breast cancer
- •3.2.1. Altered expression of death ligands and their receptors in breast cancer
- •3.2.2. Deregulation of prosurvival growth factors and their receptors
- •3.2.3. Alterations in cell adhesion and resistance to anoikis
- •3.2.4. Enhanced activation of the PI3K/AKT pathway in breast cancer
- •3.2.5. p53 inactivation in breast cancer
- •3.2.6. Altered expression of BCL-2 family of proteins in breast cancer
- •5. CONCLUSION
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2. DETECTING CELL DEATH IN THE FEMALE GONADS
- •4. APOPTOSIS AND FEMALE REPRODUCTIVE AGING
- •6. CONCLUDING REMARKS
- •REFERENCES
- •25 Apoptotic Signaling in Male Germ Cells
- •1. INTRODUCTION
- •3.1. Murine models
- •3.2. Primate models
- •3.3. Pathways of caspase activation and apoptosis
- •3.4. Apoptotic signaling in male germ cells
- •5. P38 MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) AND NITRIC OXIDE (NO)–MEDIATED INTRINSIC PATHWAY SIGNALING CONSTITUTES A CRITICAL COMPONENT OF APOPTOTIC SIGNALING IN MALE GERM CELLS AFTER HORMONE DEPRIVATION
- •11. CONCLUSIONS AND PERSPECTIVES
- •REFERENCES
- •26 Cell Death in the Cardiovascular System
- •1. INTRODUCTION
- •2. CELL DEATH IN THE VASCULATURE
- •2.1. Apoptosis in the developing blood vessels
- •2.2. Apoptosis in atherosclerosis
- •2.2.1. Vascular smooth muscle cells
- •2.2.2. Macrophages
- •2.2.3. Regulation of apoptosis in atherosclerosis
- •2.2.4. Necrosis and autophagy in atherosclerosis
- •3. CELL DEATH IN THE MYOCARDIUM
- •3.1. Cell death in myocardial infarction
- •3.1.1. Apoptosis in myocardial infarction
- •3.1.2. Necrosis in myocardial infarction
- •3.1.3. Autophagy in myocardial infarction
- •3.2. Cell death in heart failure
- •3.2.1. Apoptosis in heart failure
- •3.2.2. Necrosis in heart failure
- •3.2.3. Autophagy in heart failure
- •4. CONCLUDING REMARKS
- •ACKNOWLEDGMENTS
- •REFERENCES
- •27 Cell Death Regulation in Muscle
- •1. INTRODUCTION TO MUSCLE
- •1.1. Skeletal muscle adaptation to endurance training
- •1.2. Myonuclear domains
- •2. MITOCHONDRIALLY MEDIATED APOPTOSIS IN MUSCLE
- •2.1. Skeletal muscle apoptotic susceptibility
- •4. APOPTOSIS IN MUSCLE DURING AGING AND DISEASE
- •4.1. Aging
- •4.2. Type 2 diabetes mellitus
- •4.3. Cancer cachexia
- •4.4. Chronic heart failure
- •6. CONCLUSION
- •SUGGESTED READINGS
- •28 Cell Death in the Skin
- •1. INTRODUCTION
- •2. CELL DEATH IN SKIN HOMEOSTASIS
- •2.1. Cornification and apoptosis
- •2.2. Death receptors in the skin
- •3. CELL DEATH IN SKIN PATHOLOGY
- •3.1. Sunburn
- •3.2. Skin cancer
- •3.3. Necrolysis
- •3.4. Pemphigus
- •3.5. Eczema
- •3.6. Graft-versus-host disease
- •4. CONCLUDING REMARKS AND PERSPECTIVES
- •ACKNOWLEDGMENTS
- •SUGGESTED READINGS
- •29 Apoptosis and Cell Survival in the Immune System
- •2.1. Survival of early hematopoietic progenitors
- •2.2. Sizing of the T-cell population
- •2.2.1. Establishing central tolerance
- •2.2.2. Peripheral tolerance
- •2.2.3. Memory T cells
- •2.3. Control of apoptosis in B-cell development
- •2.3.1. Early B-cell development
- •2.3.2. Deletion of autoreactive B cells
- •2.3.3. Survival and death of activated B cells
- •3. IMPAIRED APOPTOSIS AND LEUKEMOGENESIS
- •4. CONCLUSIONS
- •ACKNOWLEDGMENTS
- •REFERENCES
- •30 Cell Death Regulation in the Hematopoietic System
- •1. INTRODUCTION
- •2. HEMATOPOIETIC STEM CELLS
- •4. ERYTHROPOIESIS
- •5. MEGAKARYOPOIESIS
- •6. GRANULOPOIESIS
- •7. MONOPOIESIS
- •8. CONCLUSION
- •ACKNOWLEDGMENTS
- •REFERENCES
- •31 Apoptotic Cell Death in Sepsis
- •1. INTRODUCTION
- •2. HOST INFLAMMATORY RESPONSE TO SEPSIS
- •3. CLINICAL OBSERVATIONS OF CELL DEATH IN SEPSIS
- •3.1. Sepsis-induced apoptosis
- •3.2. Necrotic cell death in sepsis
- •4.1. Central role of apoptosis in sepsis mortality: immune effector cells and gut epithelium
- •4.2. Apoptotic pathways in sepsis-induced immune cell death
- •4.3. Investigations implicating the extrinsic apoptotic pathway in sepsis
- •4.4. Investigations implicating the intrinsic apoptotic pathway in sepsis
- •5. THE EFFECT OF APOPTOSIS ON THE IMMUNE SYSTEM
- •5.1. Cellular effects of an increased apoptotic burdens
- •5.2. Network effects of selective loss of immune cell types
- •5.3. Studies of immunomodulation by apoptotic cells in other fields
- •7. CONCLUSION
- •REFERENCES
- •32 Host–Pathogen Interactions
- •1. INTRODUCTION
- •2. FROM THE PATHOGEN PERSPECTIVE
- •2.1. Commensals versus pathogens
- •2.2. Pathogen strategies to infect the host
- •3. HOST DEFENSE
- •3.1. Antimicrobial peptides
- •3.2. PRRs and inflammation
- •3.2.1. TLRs
- •3.2.2. NLRs
- •3.2.3. The Nod signalosome
- •3.2.4. The inflammasome
- •3.3. Cell death
- •3.3.1. Apoptosis and pathogen clearance
- •3.3.2. Pyroptosis
- •3.2.3. Caspase-independent cell death
- •3.2.4. Autophagy and autophagic cell death
- •4. CONCLUSIONS
- •REFERENCES
- •Part III Cell Death in Nonmammalian Organisms
- •1. PHENOTYPE AND ASSAYS OF YEAST APOPTOSIS
- •2.1. Pheromone-induced cell death
- •2.1.1. Colony growth
- •2.1.2. Killer-induced cell death
- •3. EXTERNAL STIMULI THAT INDUCE APOPTOSIS IN YEAST
- •4. THE GENETICS OF YEAST APOPTOSIS
- •5. PROGRAMMED AND ALTRUISTIC AGING
- •SUGGESTED READINGS
- •34 Caenorhabditis elegans and Apoptosis
- •1. Overview
- •2. KILLING
- •3. SPECIFICATION
- •4. EXECUTION
- •4.1. DNA degradation
- •4.2. Mitochondrial elimination
- •4.3. Engulfment
- •5. SUMMARY
- •SUGGESTED READINGS
- •35 Apoptotic Cell Death in Drosophila
- •2. DROSOPHILA CASPASES AND PROXIMAL REGULATORS
- •6. CLOSING COMMENTS
- •SUGGESTED READINGS
- •36 Analysis of Cell Death in Zebrafish
- •1. INTRODUCTION
- •2. WHY USE ZEBRAFISH TO STUDY CELL DEATH?
- •2.2. Molecular techniques to rapidly assess gene function in embryos
- •2.2.1. Studies of gene function using microinjections into early embryos
- •2.2.2. In situ hybridization and immunohistochemistry
- •2.3. Forward genetic screening
- •2.4. Drug and small-molecule screening
- •2.5. Transgenesis
- •2.6. Targeted knockouts
- •3.1. Intrinsic apoptosis
- •3.2. Extrinsic apoptosis
- •3.3. Chk-1 suppressed apoptosis
- •3.4. Anoikis
- •3.5. Autophagy
- •3.6. Necrosis
- •4. DEVELOPMENTAL CELL DEATH IN ZEBRAFISH EMBRYOS
- •5. THE P53 PATHWAY
- •6. PERSPECTIVES AND FUTURE DIRECTIONS
- •SUGGESTED READING
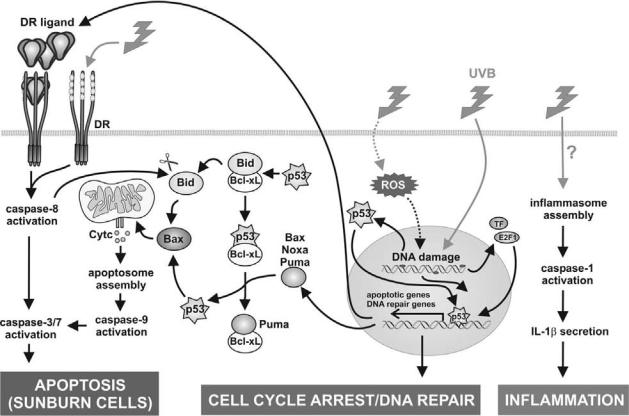
CELL DEATH IN THE SKIN |
327 |
Figure 28-3. UVB signaling in keratinocytes. UVB can lead to di erent e ects in keratinocytes, ranging from cell cycle arrest, apoptosis, and inflammasome activation. UVB radiation primarily damages nuclear DNA as a result of direct absorption and generates ROS that can induce oxidative damage to DNA and cellular proteins. The DNA damage is sensed by the DNA repair system. This leads to cytosolic stabilization of p53. In addition, other transcription factors (TF), such as NF-κB and E2F1, become activated. In the first instance, these transcription factors will drive gene expression to repair the UVB-induced damage. E2F1 is a main transcription factor stimulating DNA repair genes in keratinocytes. The p53 transcription factor mediates either cell cycle arrest or apoptosis, depending on the extent of the damage. Sustained activation of p53, together with other transcription factors, will lead to the increased expression of the proapoptotic Bcl-2 family members Puma, Bax, and Noxa, which will lead to mitochondrial damage and subsequent cytochrome c release, and of death receptors and their ligands, which will activate extrinsic apoptotic pathway. Cell surface death receptors may also be activated directly in a ligand-independent way at high doses of UV radiation. This will lead to activation of the apoptotic caspase cascade. Caspase-8 activation can cleave the proapoptotic Bid molecule to enhance its activity. However, the extrinsic pathway does not substantially contribute to the overall cell death. Surprisingly, UVB-induced apoptosis does not require de novo protein synthesis, and p53 contributes to cell death, possibly through direct interaction with the mitochondrion itself and/or with members of the Bcl-2 family. A number of cytoplasmic and mitochondrial activities of p53 have been proposed. Bid can be chased from Bcl-XL by competing p53 and induce Bax-dependent cytochrome c release. Alternatively, p53 can activate the expression of PUMA and other proapoptotic Bcl-2 family members, which then serve to release cytoplasmic p53 from the inhibitory interaction with Bcl-xL. The released p53 is then free to directly activate Bax. Bax will then induce cytochrome c release from the mitochondria. Many other signaling pathways and potential crosstalks were left out for reasons of clarity. Finally, UVB irradiation can also lead to inflammasome assembly and caspase-1 activation. However, the molecular mechanism leading to inflammasome activation is still unclear. See Color Plate 33.
3. CELL DEATH IN SKIN PATHOLOGY
3.1. Sunburn
Although CE formation does not occur through classical apoptosis, all components required for apoptosis are present in keratinocytes. One of the major environmen-
tal insults to which our skin is exposed is ultraviolet radiation (UVR), a potent agent that causes DNA damage and apoptotic sunburn cells. UV-induced apoptosis is a complex cellular process that involves many players, which are discussed next (Figure 28-3).
UVB (290–320 nm) can be directly absorbed by DNA, resulting in the formation of photo lesions such as
328 |
SASKIA LIPPENS, ESTHER HOSTE, PETER VANDENABEELE, AND WIM DECLERCQ |
cyclobutane-pyrimidine dimers (CPDs) and pyrimidine (6–4) pyrimidone. UVA radiation (>320 nm) penetrates deeply into the skin, and the genotoxic effects are mainly mediated by accumulation of reactive oxygen species (ROS) generated by activation of photosensitizers (riboflavin, porphyrins, quinones). ROS production is also excessive in UVB-irradiated skin and contributes to apoptosis, as witnessed by the protective effect of antioxidants. Most of the generated photoproducts are removed by the cellular DNA excision repair. If not, permanent mutations can lead to cancer development. Upon excessive DNA damage, the cell will undergo apoptosis, resulting in the formation of so-called sunburn cells, characterized by the presence of photo lesions, pyknotic nuclei, and eosinophilic cytoplasm.
An important player in UVB-induced apoptosis is the transcription factor p53. p53 is involved in a plethora of cellular functions, such as cell cycle arrest and activation of apoptosis, and is generally considered a tumor suppressor. By activating apoptosis, p53 protects the organism against accumulation of damaged cells that may develop into cancerous cells. The choice between cellular responses depends on the type of cell and stress and action of p53 co-activators, such as p300, CREBbinding protein (CBP), and P300/CBP-associated factor (PCAF). As mentioned previously, UVB can induce severe DNA damage. Upon DNA damage, MDM2, a p53-binding ubiquitinating enzyme that targets p53 for degradation, is inactivated, and as a consequence, p53 becomes stabilized. The important role of p53 in UVinduced apoptosis is demonstrated by the significant reduction in sunburn cells in p53 knockout mice after UVB irradiation. p53 is able to induce apoptosis through transcription-dependent or -independent mechanisms and can occur through the extrinsic and intrinsic mitochondrial apoptotic pathway. Cytoplasmic p53 can bind directly to several antiapoptotic members of the Bcl- 2 superfamily, such as Bcl-2 and Bcl-XL, thereby neutralizing their antiapoptotic activity and acting itself as a proapoptotic protein. Nuclear p53 can transactivate proapoptotic Bcl-2 family members, such as Bax, p53upregulated modulator of apoptosis (PUMA), Bik/Nbk, and Noxa. In Noxa-deficient animals, UV-induced keratinocyte apoptosis is suppressed, whereas this is not the case in PUMA-deficient mice. This is consistent with the observation that the UV-induced Noxa levels are far higher than the PUMA levels. Mouse embryonal fibroblasts deficient for both caspase-3 and -7 are also resistant to UVB-induced cell death. This may imply the existence of a positive feedback loop between pre- (e.g., Noxa) and postmitochondrial (caspases) signaling molecules required for UVB-induced cell death.
Among the transcriptional targets of p53, there are several genes involved in ROS generation, whereas other p53-dependent genes have an antioxidant function, suggesting a role for p53 in controlling ROS levels in homeostatic and pathogenic conditions. Interestingly, p53 also protects the skin by augmenting skin tanning upon UV irradiation. It was shown that p53 regulates induction of pro-opiomelanocortin–derived bioactive peptides such as α-melanocyte-stimulating hormone, adrenocorticotropic hormone, and β-endorphin, which are key players in melanin production and secretion.
The DNA repair-promoting transcription factor E2F1, which is upregulated after DNA damage, is also involved in UV-induced apoptosis. In several cellular systems, the proapoptotic potential of E2F1 has been demonstrated. However, in vivo data suggest that the function of E2F1 in skin is mainly antiapoptotic. Its in vivo antiapoptotic potential is clear from mice over-expressing E2F1 in the skin. In accordance, E2F1-deficient mice exhibit a higher abundance of sunburn cells upon UVR. E2F1−/– p53−/– double-knockout mice exhibit the elevated UVB-induced apoptosis, which is seen in E2F1 single-knockout animals. This implies that E2F1 can function in a p53-independent way. Chronic exposure to UVR can induce inactivating mutations in the p53 gene and thereby hamper its potency to induce apoptosis, making cells more prone to malignant transformation.
Two other members of the p53 family, p63 and p73, are capable of transactivating p53 target genes and have been implicated in the regulation of apoptosis. Mice deficient for p63 have no epidermis or other squamous epithelia and lack epithelial appendages. There is some debate concerning the mechanism by which p63 exerts its function in skin. It has been thought to mainly play a role in stem cell proliferation; others claim a role in epidermal lineage commitment. Studying p63 in skin differentiation is complicated by the fact that the p63 gene is transcribed into 10 isoforms, either containing (p63TA isoforms) or lacking the transactivation domain ( Np63 isoforms). A mouse model over-expressing Np63α specifically in the epidermis showed a reduction in UVBinduced skin apoptosis, probably as a result of competing with p53-dependent signaling. Interestingly, it has been demonstrated that in human keratinocytes deficient in p53 and p63, there is impaired repair of CPDs. In these cells there was a marked decrease observed in the expression of DDB2 and XPC, which are key DNA damage-recognition proteins. p73-deficient mice show, in contrast to p53-deficient mice, no increased spontaneous tumorigenesis, but exhibit neurologic defects; no skin abnormalities have been described.
CELL DEATH IN THE SKIN |
329 |
Involvement of the intrinsic pathway upon UVBinduced keratinocyte apoptosis has been shown by inhibitor studies targeting caspase-9. In accordance with this, it has been shown that over-expression of Bcl-2 renders keratinocytes resistant to UVB-induced apoptosis. Expression of Apaf-1 is increased upon UV irradiation of keratinocytes. Caspase cleavage of PKCδ is required for UVB-induced apoptosis. The generated PKCδ catalytic fragment translocates to the mitochondria, targets antiapoptotic Mcl-1 for degradation, and triggers redistribution and activation of Bax. Besides apoptotic caspases, UVR can also activate inflammatory caspases, which are activated in inflammasome complexes. UV-irradiated human keratinocytes have been shown to secrete interleukin-1β, a caspase-1 substrate, in an inflammasome-dependent manner. This makes the skin an important component of innate immunity, which is logical, given the fact that keratinocytes are continuously exposed to environmental stressors. When mice deficient for caspase-1 are irradiated with UVB, the number of infiltrated neutrophils is strongly reduced as compared with that of wild-type mice. How UVB is able to induce assembly and activation of the inflammasome is currently unknown. In cultured keratinocytes, the activation of caspase-1 is dependent on an increase in cytoplasmic Ca2+ concentration. Interestingly, UV irradiation of human epidermal keratinocytes results in an increase in intracellular Ca2+ concentration.
The skin of caspase-14 deficient mice is highly sensitive to UVB irradiation, which is characterized by increased UVB-induced CPDs and consequent keratinocyte apoptosis. This is probably due to a reduced UV scavenging effect of the stratum corneum. It is of note that filaggrin degradation, which gives rise to NMFs and urocanic acid, a major absorber of UVB, is impaired in caspase-14 deficient mice. However, the exact mechanism by which caspase-14 protects against UVB-induced apoptosis in the skin remains to be determined. The implication of caspase-14 in UVB protection may be reflected in the fact that caspase-14 has thus far been found only in terrestrial mammals.
DRs are also involved in UV-induced apoptosis, and their triggering can occur in ligand-independent and ligand-dependent ways. Ligand-independent UVBinduced clustering of the DRs Fas and TNF-R1 has been described in human keratinocyte cultures. However, UVB irradiation can also induce upregulation of DRs and its ligands, such as Fas and FasL and TNF. In accordance with this, mice that are deficient for FasL or TNF-R1 show a reduction in the occurrence of sunburn cell formation.
3.2. Skin cancer
An imbalance toward too little apoptosis or too much cell survival in the epidermis can result in skin tumor formation. Epidermal tumors are divided into melanoma and nonmelanoma skin cancers (NMSCs). The majority of NMSCs (80%) are basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs), both arising from cutaneous keratinocytes. Apoptosis is a major cancer defense system in the skin, and generally, triggering or resensitizing of the apoptotic pathway is used in different anticancer treatments. Mutations in the p53 gene, induced by chemical or UVB exposure, are probably an early step during tumorigenesis in BCC and SCC. Mice deficient in p53 are very sensitive to UV-induced SCC formation, and skin-specific transgenes for the p53 regulator MDM2 inhibit UV induction of p53 and are more susceptible to chemical carcinogenesis.
In correlation with their elevated survival status, tumors often have an altered expression of antior proapoptotic proteins. Cancer treatment often aims to restore or invert this imbalance to make use of the apoptotic program to kill the tumor.
For instance, Fas levels are decreased in BCC and in melanomas, probably as a result of oncogenic Ras. Certain melanomas even have inactivating mutations in the death domain of Fas. Increase in Fas expression levels is accomplished by different anticancer drugs and cytokines. In addition, TRAIL-R activated cell death by antibodies or recombinant TRAIL appears promising as a cancer therapy. Interestingly, cancer cell lines, including melanomas, are sensitive to TRAIL killing, whereas nontransformed cells are not, therefore reducing the possible side effects of TRAIL therapy.
Tumors can sometimes escape from apoptosis because of increased levels of antiapoptotic proteins. First, higher levels of FLICE-like inhibitory protein (FLIP) allow melanomas to escape from DR-induced apoptosis by conventional T cells. Second, the inhibitor of apoptosis (IAP) family member survivin is not expressed in normal skin, but it is found in NMSCs. Third, tumor resistance to cytotoxic agents and radiotherapy is associated with increased expression of antiapoptotic Bcl-2 family members such as Bcl-XL and Bcl-2. In certain melanomas, low or absence of sensitivity to TRAIL-induced apoptosis can be overcome by knocking down FLIP, survivin, Bcl-2, or IAPs. One strategy to lower Bcl-2 levels in tumors is the use of antisense oligonucleotides, called oblimersen. However, this approach failed in clinical trials for melanoma (and other cancers) treatment. A promising alternative are
330 |
SASKIA LIPPENS, ESTHER HOSTE, PETER VANDENABEELE, AND WIM DECLERCQ |
synthetic BH3-only peptides or small organic molecules that interact with the hydrophobic cleft of antiapoptotic Bcl-2 family members and that, as such, are able to induce apoptosis. An alternative therapy making use of IAP inhibitors is currently under development.
Instead of decreased sensitivity to apoptosis, overactivation of survival pathways can also be the basis of tumorigenesis in the skin. Transgenic mice that over-express Akt in basal keratinocytes, which leads both to increased proliferation and decreased apoptosis, develop spontaneous tumors and show increased sensitivity to tissue plasminogen activator (TPA)–induced carcinogenesis. Other survival pathways, such as the MAPK pathway, which controls cell growth and differentiation, are also important in skin tumorigenesis. However, these pathways can result in oncogenic or tumor-suppressor effects, depending on the cellular status. For example, the activator protein-1 transcription factor can promote or suppress skin tumor formation depending on its subunit composition.
As discussed previously (see Section 2, Cell Death in Skin Homeostasis), NF-κB signaling plays a crucial role in skin homeostasis. The role of NF-κB in skin tumorigenesis is diverse; both activation or inactivation can be advantageous for cancer development. The tumor-promoting role of NF-κB is demonstrated in familial cylindromatosis. This disease is caused by loss-of-function mutations in cylindromatosis (CYLD), a deubiquitinase responsible for dampening of NF-κB activation. Hence absence of CYLD will result in overactivation of NF-κB. Similar to human cylindromatosis, mice lacking CYLD are more sensitive to TPA-induced tumor formation, underscoring the importance of NF- κB activation in the development of certain types of skin cancer. In SCC, Ras activity is often increased as a result of mutations in the gene itself that render it constitutively active. Cell cycle arrest induced by oncogenic Ras can be overcome by blocking NF-κB, as shown by IkBα overexpression. So here NF-κB performs tumorsuppressor activity.
3.3. Necrolysis
Toxic epidermal necrolysis (TEN; Lyell’s syndrome) and Stevens-Johnson syndrome (SJS) are rare acute dermatological diseases defined by epidermal necrosis and mucosal erosions, with large areas of loss of contact between epidermis and dermis and massive keratinocyte apoptosis. In SJS and TEN, up to 10% and 30%, respectively, of skin surface can be detached. The main cause is a severe adverse drug reaction, or
in some cases, bacterial infection. The effector cells of the disease are thought to be drug-specific cytotoxic T cells, but the pathophysiologic mechanisms are largely unknown. So far, no specific treatment exists. However, FasL expression is increased in lesional skin keratinocytes of TEN patients. Interestingly, Fassensitive Jurkat cells undergo Fas-dependent apoptosis when incubated on skin cryosections of TEN patients. In addition, the therapeutic potential of intravenous administration of human intravenously collected immunoglobulins from healthy donors, also containing neutralizing anti-Fas antibodies, has been demonstrated in TEN patients. These results suggest an important role of the Fas signaling pathway in the development of TEN. SJS and TEN were long believed to belong to a spectrum of disorders that included erythema multiforme majus (EMM); however, now SJS and TEN are considered to be different diseases than EMM. In EMM, skin eruptions are mainly found on the extremities, whereas in TEN, blistering is widespread. In EMM, mucosal lesions may occur, but are mostly limited to the oral cavity, whereas in TEN, there are severe mucosal erosions. Finally, in TEN the mortality rate is much higher than in EMM.
3.4. Pemphigus
Pemphigus diseases are autoimmune cutaneous blistering disorders characterized by the presence of autoantibodies against structural proteins of the intracellular junctions.
Apoptotic keratinocytes are present in lesional tissue of pemphigus vulgaris (PV) patients. When PV immunoglobulin autoantibodies are added to keratinocytes in vitro, FasL is secreted, reduction of Bcl-2 is observed, and several apoptotic proteins are upregulated. Similar results were obtained after addition of an antibody against Fas Receptor to cultured keratinocytes. These data point to a possible involvement of the extrinsic apoptotic pathway in PV; however, it should be noted that this hypothesis has not been proven in vivo.
3.5. Eczema
The term eczematous dermatitis (eczema) comprises a group of inflammatory skin diseases, such as atopic dermatitis, and that are often characterized by the formation of vesicles associated with exudation. It has been shown that keratinocyte apoptosis is important for vesicle formation. The pathogenesis of eczema is T- cell mediated; secretion of interferon γ by these cells
