
- •Contents
- •Contributors
- •Part I General Principles of Cell Death
- •1 Human Caspases – Apoptosis and Inflammation Signaling Proteases
- •1.1. Apoptosis and limited proteolysis
- •1.2. Caspase evolution
- •2. ACTIVATION MECHANISMS
- •2.2. The activation platforms
- •2.4. Proteolytic maturation
- •3. CASPASE SUBSTRATES
- •4. REGULATION BY NATURAL INHIBITORS
- •REFERENCES
- •2 Inhibitor of Apoptosis Proteins
- •2. CELLULAR FUNCTIONS AND PHENOTYPES OF IAP
- •3. IN VIVO FUNCTIONS OF IAP FAMILY PROTEINS
- •4. SUBCELLULAR LOCATIONS OF IAP
- •8. IAP–IAP INTERACTIONS
- •10. ENDOGENOUS ANTAGONISTS OF IAP
- •11. IAPs AND DISEASE
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2.1. The CD95 (Fas/APO-1) system
- •2.1.1. CD95 and CD95L: discovery of the first direct apoptosis-inducing receptor-ligand system
- •2.1.2. Biochemistry of CD95 apoptosis signaling
- •2.2. The TRAIL (Apo2L) system
- •3.1. The TNF system
- •3.1.1. Biochemistry of TNF signal transduction
- •3.1.2. TNF and TNF blockers in the clinic
- •3.2. The DR3 system
- •4. THE DR6 SYSTEM
- •6. CONCLUDING REMARKS AND OUTLOOK
- •SUGGESTED READINGS
- •4 Mitochondria and Cell Death
- •1. INTRODUCTION
- •2. MITOCHONDRIAL PHYSIOLOGY
- •3. THE MITOCHONDRIAL PATHWAY OF APOPTOSIS
- •9. CONCLUSIONS
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •3. INHIBITING APOPTOSIS
- •4. INHIBITING THE INHIBITORS
- •6. THE BCL-2 FAMILY AND CANCER
- •SUGGESTED READINGS
- •6 Endoplasmic Reticulum Stress Response in Cell Death and Cell Survival
- •1. INTRODUCTION
- •2. THE ESR IN YEAST
- •3. THE ESR IN MAMMALS
- •4. THE ESR AND CELL DEATH
- •5. THE ESR IN DEVELOPMENT AND TISSUE HOMEOSTASIS
- •6. THE ESR IN HUMAN DISEASE
- •7. CONCLUSION
- •7 Autophagy – The Liaison between the Lysosomal System and Cell Death
- •1. INTRODUCTION
- •2. AUTOPHAGY
- •2.2. Physiologic functions of autophagy
- •2.3. Autophagy and human pathology
- •3. AUTOPHAGY AND CELL DEATH
- •3.1. Autophagy as anti–cell death mechanism
- •3.2. Autophagy as a cell death mechanism
- •3.3. Molecular players of the autophagy–cell death cross-talk
- •4. AUTOPHAGY, CELLULAR DEATH, AND CANCER
- •5. CONCLUDING REMARKS AND PENDING QUESTIONS
- •SUGGESTED READINGS
- •8 Cell Death in Response to Genotoxic Stress and DNA Damage
- •1. TYPES OF DNA DAMAGE AND REPAIR SYSTEMS
- •2. DNA DAMAGE RESPONSE
- •2.2. Transducers
- •2.3. Effectors
- •4. CHROMATIN MODIFICATIONS
- •5. CELL CYCLE CHECKPOINT REGULATION
- •6. WHEN REPAIR FAILS: SENESCENCE VERSUS APOPTOSIS
- •6.1. DNA damage response and the induction of apoptosis
- •6.2. p53-independent mechanisms of apoptosis
- •6.3. DNA damage response and senescence induction
- •7. DNA DAMAGE FROM OXIDATIVE STRESS
- •SUGGESTED READINGS
- •9 Ceramide and Lipid Mediators in Apoptosis
- •1. INTRODUCTION
- •3.1. Basic cell signaling often involves small molecules
- •3.2. Sphingolipids are cell-signaling molecules
- •3.2.1. Ceramide induces apoptosis
- •3.2.2. Ceramide accumulates during programmed cell death
- •3.2.3. Inhibition of ceramide production alters cell death signaling
- •4.1. Ceramide is generated through SM hydrolysis
- •4.3. aSMase can be activated independently of extracellular receptors to regulate apoptosis
- •4.4. Controversial aspects of the role of aSMase in apoptosis
- •4.5. De novo ceramide synthesis regulates programmed cell death
- •4.6. p53 and Bcl-2–like proteins are connected to de novo ceramide synthesis
- •4.7. The role and regulation of de novo synthesis in ceramide-mediated cell death is poorly understood
- •5. CONCLUDING REMARKS AND FUTURE DIRECTIONS
- •5.1. Who? (Which enzyme?)
- •5.2. What? (Which ceramide?)
- •5.3. Where? (Which compartment?)
- •5.4. When? (At what steps?)
- •5.5. How? (Through what mechanisms?)
- •5.6. What purpose?
- •6. SUMMARY
- •SUGGESTED READINGS
- •1. General Introduction
- •1.1. Cytotoxic lymphocytes and apoptosis
- •2. CYTOTOXIC GRANULES AND GRANULE EXOCYTOSIS
- •2.1. Synthesis and loading of the cytotoxic granule proteins into the secretory granules
- •2.2. The immunological synapse
- •2.3. Secretion of granule proteins
- •2.4. Uptake of proapoptotic proteins into the target cell
- •2.5. Activation of death pathways by granzymes
- •3. GRANULE-BOUND CYTOTOXIC PROTEINS
- •3.1. Perforin
- •3.2. Granulysin
- •3.3. Granzymes
- •3.3.1. GrB-mediated apoptosis
- •3.3.2. GrA-mediated cell death
- •3.3.3. Orphan granzyme-mediated cell death
- •5. CONCLUSIONS
- •REFERENCES
- •Part II Cell Death in Tissues and Organs
- •1.1. Death by trophic factor deprivation
- •1.2. Key molecules regulating neuronal apoptosis during development
- •1.2.1. Roles of caspases and Apaf-1 in neuronal cell death
- •1.2.2. Role of Bcl-2 family members in neuronal cell death
- •1.3. Signal transduction from neurotrophins and neurotrophin receptors
- •1.3.1. Signals for survival
- •1.3.2. Signals for death
- •2.1. Apoptosis in neurodegenerative diseases
- •2.1.4. Amyotrophic lateral sclerosis
- •2.2. Necrotic cell death in neurodegenerative diseases
- •2.2.1. Calpains
- •2.2.2. Cathepsins
- •3. CONCLUSIONS
- •ACKNOWLEDGMENT
- •SUGGESTED READINGS
- •ACKNOWLEDGMENT
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •5. S-NITROSYLATION OF PARKIN
- •7. POTENTIAL TREATMENT OF EXCESSIVE NMDA-INDUCED Ca2+ INFLUX AND FREE RADICAL GENERATION
- •8. FUTURE THERAPEUTICS: NITROMEMANTINES
- •9. CONCLUSIONS
- •Acknowledgments
- •SUGGESTED READINGS
- •3. MITOCHONDRIAL PERMEABILITY TRANSITION ACTIVATED BY Ca2+ AND OXIDATIVE STRESS
- •4.1. Mitochondrial apoptotic pathways
- •4.2. Bcl-2 family proteins
- •4.3. Caspase-dependent apoptosis
- •4.4. Caspase-independent apoptosis
- •4.5. Calpains in ischemic neural cell death
- •5. SUMMARY
- •ACKNOWLEDGMENTS
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2. HISTORICAL ANTECEDENTS
- •7.1. Activation of p21 waf1/cip1: Targeting extrinsic and intrinsic pathways to death
- •8. CONCLUSION
- •ACKNOWLEDGMENTS
- •REFERENCES
- •16 Apoptosis and Homeostasis in the Eye
- •1.1. Lens
- •1.2. Retina
- •2. ROLE OF APOPTOSIS IN DISEASES OF THE EYE
- •2.1. Glaucoma
- •2.2. Age-related macular degeneration
- •4. APOPTOSIS AND OCULAR IMMUNE PRIVILEGE
- •5. CONCLUSIONS
- •SUGGESTED READINGS
- •17 Cell Death in the Inner Ear
- •3. THE COCHLEA IS THE HEARING ORGAN
- •3.1. Ototoxic hair cell death
- •3.2. Aminoglycoside-induced hair cell death
- •3.3. Cisplatin-induced hair cell death
- •3.4. Therapeutic strategies to prevent hair cell death
- •3.5. Challenges to studies of hair cell death
- •4. SPIRAL GANGLION NEURON DEATH
- •4.1. Neurotrophic support from sensory hair cells and supporting cells
- •4.2. Afferent activity from hair cells
- •4.3. Molecular manifestations of spiral ganglion neuron death
- •4.4. Therapeutic interventions to prevent SGN death
- •ACKNOWLEDGMENTS
- •SUGGESTED READINGS
- •18 Cell Death in the Olfactory System
- •1. Introduction
- •2. Anatomical Aspects
- •3. Life and Death in the Olfactory System
- •3.1. Olfactory epithelium
- •3.2. Olfactory bulb
- •REFERENCES
- •1. Introduction
- •3.1. Beta cell death in the development of T1D
- •3.2. Mechanisms of beta cell death in type 1 diabetes
- •3.2.1. Apoptosis signaling pathways downstream of death receptors and inflammatory cytokines
- •3.2.2. Oxidative stress
- •3.3. Mechanisms of beta cell death in type 2 diabetes
- •3.3.1. Glucolipitoxicity
- •3.3.2. Endoplasmic reticulum stress
- •5. SUMMARY
- •Acknowledgments
- •REFERENCES
- •20 Apoptosis in the Physiology and Diseases of the Respiratory Tract
- •1. APOPTOSIS IN LUNG DEVELOPMENT
- •2. APOPTOSIS IN LUNG PATHOPHYSIOLOGY
- •2.1. Apoptosis in pulmonary inflammation
- •2.2. Apoptosis in acute lung injury
- •2.3. Apoptosis in chronic obstructive pulmonary disease
- •2.4. Apoptosis in interstitial lung diseases
- •2.5. Apoptosis in pulmonary arterial hypertension
- •2.6. Apoptosis in lung cancer
- •SUGGESTED READINGS
- •21 Regulation of Cell Death in the Gastrointestinal Tract
- •1. INTRODUCTION
- •2. ESOPHAGUS
- •3. STOMACH
- •4. SMALL AND LARGE INTESTINE
- •5. LIVER
- •6. PANCREAS
- •7. SUMMARY AND CONCLUDING REMARKS
- •SUGGESTED READINGS
- •22 Apoptosis in the Kidney
- •1. NORMAL KIDNEY STRUCTURE AND FUNCTION
- •3. APOPTOSIS IN ADULT KIDNEY DISEASE
- •4. REGULATION OF APOPTOSIS IN KIDNEY CELLS
- •4.1. Survival factors
- •4.2. Lethal factors
- •4.2.1. TNF superfamily cytokines
- •4.2.2. Other cytokines
- •4.2.3. Glucose
- •4.2.4. Drugs and xenobiotics
- •4.2.5. Ischemia-reperfusion and sepsis
- •5. THERAPEUTIC APPROACHES
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2. APOPTOSIS IN THE NORMAL BREAST
- •2.1. Occurrence and role of apoptosis in the developing breast
- •2.2.2. Death ligands and death receptor pathway
- •2.2.4. LIF-STAT3 proapoptotic signaling
- •2.2.5. IGF survival signaling
- •2.2.6. Regulation by adhesion
- •2.2.7. PI3K/AKT pathway: molecular hub for survival signals
- •2.2.8. Downstream regulators of apoptosis: the BCL-2 family members
- •3. APOPTOSIS IN BREAST CANCER
- •3.1. Apoptosis in breast tumorigenesis and cancer progression
- •3.2. Molecular dysregulation of apoptosis in breast cancer
- •3.2.1. Altered expression of death ligands and their receptors in breast cancer
- •3.2.2. Deregulation of prosurvival growth factors and their receptors
- •3.2.3. Alterations in cell adhesion and resistance to anoikis
- •3.2.4. Enhanced activation of the PI3K/AKT pathway in breast cancer
- •3.2.5. p53 inactivation in breast cancer
- •3.2.6. Altered expression of BCL-2 family of proteins in breast cancer
- •5. CONCLUSION
- •SUGGESTED READINGS
- •1. INTRODUCTION
- •2. DETECTING CELL DEATH IN THE FEMALE GONADS
- •4. APOPTOSIS AND FEMALE REPRODUCTIVE AGING
- •6. CONCLUDING REMARKS
- •REFERENCES
- •25 Apoptotic Signaling in Male Germ Cells
- •1. INTRODUCTION
- •3.1. Murine models
- •3.2. Primate models
- •3.3. Pathways of caspase activation and apoptosis
- •3.4. Apoptotic signaling in male germ cells
- •5. P38 MITOGEN-ACTIVATED PROTEIN KINASE (MAPK) AND NITRIC OXIDE (NO)–MEDIATED INTRINSIC PATHWAY SIGNALING CONSTITUTES A CRITICAL COMPONENT OF APOPTOTIC SIGNALING IN MALE GERM CELLS AFTER HORMONE DEPRIVATION
- •11. CONCLUSIONS AND PERSPECTIVES
- •REFERENCES
- •26 Cell Death in the Cardiovascular System
- •1. INTRODUCTION
- •2. CELL DEATH IN THE VASCULATURE
- •2.1. Apoptosis in the developing blood vessels
- •2.2. Apoptosis in atherosclerosis
- •2.2.1. Vascular smooth muscle cells
- •2.2.2. Macrophages
- •2.2.3. Regulation of apoptosis in atherosclerosis
- •2.2.4. Necrosis and autophagy in atherosclerosis
- •3. CELL DEATH IN THE MYOCARDIUM
- •3.1. Cell death in myocardial infarction
- •3.1.1. Apoptosis in myocardial infarction
- •3.1.2. Necrosis in myocardial infarction
- •3.1.3. Autophagy in myocardial infarction
- •3.2. Cell death in heart failure
- •3.2.1. Apoptosis in heart failure
- •3.2.2. Necrosis in heart failure
- •3.2.3. Autophagy in heart failure
- •4. CONCLUDING REMARKS
- •ACKNOWLEDGMENTS
- •REFERENCES
- •27 Cell Death Regulation in Muscle
- •1. INTRODUCTION TO MUSCLE
- •1.1. Skeletal muscle adaptation to endurance training
- •1.2. Myonuclear domains
- •2. MITOCHONDRIALLY MEDIATED APOPTOSIS IN MUSCLE
- •2.1. Skeletal muscle apoptotic susceptibility
- •4. APOPTOSIS IN MUSCLE DURING AGING AND DISEASE
- •4.1. Aging
- •4.2. Type 2 diabetes mellitus
- •4.3. Cancer cachexia
- •4.4. Chronic heart failure
- •6. CONCLUSION
- •SUGGESTED READINGS
- •28 Cell Death in the Skin
- •1. INTRODUCTION
- •2. CELL DEATH IN SKIN HOMEOSTASIS
- •2.1. Cornification and apoptosis
- •2.2. Death receptors in the skin
- •3. CELL DEATH IN SKIN PATHOLOGY
- •3.1. Sunburn
- •3.2. Skin cancer
- •3.3. Necrolysis
- •3.4. Pemphigus
- •3.5. Eczema
- •3.6. Graft-versus-host disease
- •4. CONCLUDING REMARKS AND PERSPECTIVES
- •ACKNOWLEDGMENTS
- •SUGGESTED READINGS
- •29 Apoptosis and Cell Survival in the Immune System
- •2.1. Survival of early hematopoietic progenitors
- •2.2. Sizing of the T-cell population
- •2.2.1. Establishing central tolerance
- •2.2.2. Peripheral tolerance
- •2.2.3. Memory T cells
- •2.3. Control of apoptosis in B-cell development
- •2.3.1. Early B-cell development
- •2.3.2. Deletion of autoreactive B cells
- •2.3.3. Survival and death of activated B cells
- •3. IMPAIRED APOPTOSIS AND LEUKEMOGENESIS
- •4. CONCLUSIONS
- •ACKNOWLEDGMENTS
- •REFERENCES
- •30 Cell Death Regulation in the Hematopoietic System
- •1. INTRODUCTION
- •2. HEMATOPOIETIC STEM CELLS
- •4. ERYTHROPOIESIS
- •5. MEGAKARYOPOIESIS
- •6. GRANULOPOIESIS
- •7. MONOPOIESIS
- •8. CONCLUSION
- •ACKNOWLEDGMENTS
- •REFERENCES
- •31 Apoptotic Cell Death in Sepsis
- •1. INTRODUCTION
- •2. HOST INFLAMMATORY RESPONSE TO SEPSIS
- •3. CLINICAL OBSERVATIONS OF CELL DEATH IN SEPSIS
- •3.1. Sepsis-induced apoptosis
- •3.2. Necrotic cell death in sepsis
- •4.1. Central role of apoptosis in sepsis mortality: immune effector cells and gut epithelium
- •4.2. Apoptotic pathways in sepsis-induced immune cell death
- •4.3. Investigations implicating the extrinsic apoptotic pathway in sepsis
- •4.4. Investigations implicating the intrinsic apoptotic pathway in sepsis
- •5. THE EFFECT OF APOPTOSIS ON THE IMMUNE SYSTEM
- •5.1. Cellular effects of an increased apoptotic burdens
- •5.2. Network effects of selective loss of immune cell types
- •5.3. Studies of immunomodulation by apoptotic cells in other fields
- •7. CONCLUSION
- •REFERENCES
- •32 Host–Pathogen Interactions
- •1. INTRODUCTION
- •2. FROM THE PATHOGEN PERSPECTIVE
- •2.1. Commensals versus pathogens
- •2.2. Pathogen strategies to infect the host
- •3. HOST DEFENSE
- •3.1. Antimicrobial peptides
- •3.2. PRRs and inflammation
- •3.2.1. TLRs
- •3.2.2. NLRs
- •3.2.3. The Nod signalosome
- •3.2.4. The inflammasome
- •3.3. Cell death
- •3.3.1. Apoptosis and pathogen clearance
- •3.3.2. Pyroptosis
- •3.2.3. Caspase-independent cell death
- •3.2.4. Autophagy and autophagic cell death
- •4. CONCLUSIONS
- •REFERENCES
- •Part III Cell Death in Nonmammalian Organisms
- •1. PHENOTYPE AND ASSAYS OF YEAST APOPTOSIS
- •2.1. Pheromone-induced cell death
- •2.1.1. Colony growth
- •2.1.2. Killer-induced cell death
- •3. EXTERNAL STIMULI THAT INDUCE APOPTOSIS IN YEAST
- •4. THE GENETICS OF YEAST APOPTOSIS
- •5. PROGRAMMED AND ALTRUISTIC AGING
- •SUGGESTED READINGS
- •34 Caenorhabditis elegans and Apoptosis
- •1. Overview
- •2. KILLING
- •3. SPECIFICATION
- •4. EXECUTION
- •4.1. DNA degradation
- •4.2. Mitochondrial elimination
- •4.3. Engulfment
- •5. SUMMARY
- •SUGGESTED READINGS
- •35 Apoptotic Cell Death in Drosophila
- •2. DROSOPHILA CASPASES AND PROXIMAL REGULATORS
- •6. CLOSING COMMENTS
- •SUGGESTED READINGS
- •36 Analysis of Cell Death in Zebrafish
- •1. INTRODUCTION
- •2. WHY USE ZEBRAFISH TO STUDY CELL DEATH?
- •2.2. Molecular techniques to rapidly assess gene function in embryos
- •2.2.1. Studies of gene function using microinjections into early embryos
- •2.2.2. In situ hybridization and immunohistochemistry
- •2.3. Forward genetic screening
- •2.4. Drug and small-molecule screening
- •2.5. Transgenesis
- •2.6. Targeted knockouts
- •3.1. Intrinsic apoptosis
- •3.2. Extrinsic apoptosis
- •3.3. Chk-1 suppressed apoptosis
- •3.4. Anoikis
- •3.5. Autophagy
- •3.6. Necrosis
- •4. DEVELOPMENTAL CELL DEATH IN ZEBRAFISH EMBRYOS
- •5. THE P53 PATHWAY
- •6. PERSPECTIVES AND FUTURE DIRECTIONS
- •SUGGESTED READING

92 |
THOMAS D. MULLEN, RUSSELL W. JENKINS, LINA M. OBEID, AND YUSUF A. HANNUN |
Box 9-2. Methods of sphingolipid analysis II: High-performance liquid chromatography and mass spectrometry
The introduction of high-performance liquid chromatography (HPLC) coupled with mass spectrometry (LC/MS) to the measurement of sphingolipid levels has allowed investigators to study ceramide and other sphingolipids in greater detail. In this method, lipid samples and standards are separated using reverse phase chromatography, and fractions are analyzed by mass spectrometry. Individual sphingolipids are identified by both retention time in the column as well as their mass transitions, and, using calibration standards, the sphingolipids may be quantified. The advantage of LC/MS lies in the ability to simultaneously quantify multiple sphingolipid species (e.g., ceramide, sphingosine, and S1P) as well as ceramide subspecies with particular acyl chain lengths (e.g., C16-ceramide vs. C18-ceramide).
Using this technology, it has been found that certain subspecies of ceramide (e.g., C16-ceramide, C18-ceramide, C24:1-ceramide) may di erentially regulate cell death. After induction of apoptosis, ceramide species accumulate at different rates or to di ering degrees, and some studies suggest that certain species, but not others, are associated with the promotion of apoptosis. In head and neck cancer cell lines, for example, the production of C18-ceramide by CerS1 was linked to caspase activation and cell death, whereas C16-ceramide, a product of CerS5 and CerS6, failed to exhibit the same association. Although the significance of these findings remains a matter of speculation, the data suggest that there may be specific roles for individual ceramide species in regulating cell death.
|
100 |
|
|
|
|
|
C24-Cer |
|
|
|
|
|
|
|
|
|
|
|
90 |
|
|
|
|
|
|
|
Abundance |
80 |
|
|
|
|
C24:1-Cer |
|
|
70 |
|
|
|
|
|
|
||
C17-Sph |
|
|
|
|
|
|
||
60 |
C |
/C |
24 |
-Cer |
|
|
||
|
17 |
|
|
|
|
|||
|
|
|
|
|
|
|
||
50 |
|
C17/C18-Cer |
|
|
||||
Relative |
40 |
|
C16-Cer |
|
|
|
||
30 |
|
C17/C16-Cer |
C |
-Cer |
||||
|
|
C17-dHSph |
|
|
|
26 |
|
|
|
20 |
|
|
|
|
|
||
|
10 |
|
C13/C16-Cer |
|
|
|||
|
C17-S1P |
|
|
|
|
|
|
|
|
0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
0 |
5 |
10 |
15 |
20 |
25 |
30 |
|
Figure B9-2. LC/MS detection of ceramide species. Example chromatogram of ceramides and sphingoid bases extracted from MCF-7 adenocarcinoma cells. Unnatural, synthetic sphingolipid species containing C13- or C17-sphingoid bases (e.g., C17/C16-ceramide) are used as internal standards for HPLC separation. Most mammalian ceramides contain C18-sphingoid bases and are therefore distinguishable from the synthetic standards by their retention time as well as mass transition. Using ceramide standards to generate calibration curves, ceramides are quantified and normalized to total protein or total lipid phosphate.
Time (min)
reception of a stimulus, the production and activation of signaling intermediates, the activation of effectors, and a resulting change in cell behavior (Figure 9-3). In the case of programmed cell death, there is a multitude of potential stimuli, and although the “reception” phase of cell death signaling may be divergent in response to various stimuli, at some level many signaling pathways converge to produce a seemingly unified response – apoptosis. A number of proapoptotic signaling modalities are engaged to bring about apoptosis, including Ca2+ release, production of lipid mediators, and the activation of proteases.
3.1. Basic cell signaling often involves small molecules
Most cellular signaling uses small molecules to convey information between protein components of each pathway. For example, Ca2+ signaling can occur by
releasing Ca2+ from compartments such as the ER or extracellular milieu where Ca2+ concentrations are relatively high. After stimulation and activation of Ca2+ channels, elevations in cytosolic Ca2+ can cause the modulation of multiple downstream effectors. In a similar fashion, signaling sphingolipids such as ceramide can be released from “storage” in the form of SM at the plasma membrane to produce local changes in the concentration of ceramide. However, unlike soluble free Ca2+ , the hydrophobic nature of ceramide confines it to the membrane, where it can interact with membrane proteins (e.g., receptor signaling complexes) or soluble proteins that might be recruited from the cytosol (e.g., protein phosphatases).
3.2. Sphingolipids are cell-signaling molecules
The study of sphingolipids as signaling molecules originally emerged from the finding that sphingosine inhibits

CERAMIDE AND LIPID MEDIATORS IN APOPTOSIS |
93 |
Stimulus |
|
|
Endothelin |
TNF-α |
|||
Receptor |
|
|
|
|
|
|
|
Endothelin receptor |
TNFR |
||||||
|
|
|
(GPCR) |
||||
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
G proteins |
Adapter |
|||
Signaling |
|
|
Proteins |
||||
|
|
|
|
|
|||
intermediates |
|
|
|
|
|
|
|
|
|
|
PLC |
aSMase |
|||
|
|
|
|
||||
Small molecule |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
||
|
DAG + IP3 |
Ceramide |
|||||
messengers |
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PKC activation |
Receptor |
|||
Effectors |
|
|
|
and |
|||
|
|
|
clustering and |
||||
|
|
Ca |
2+ |
|
|||
|
|
|
|
||||
|
|
|
channel |
signaling |
|||
|
|
|
opening |
||||
|
|
|
|
|
|
|
|
Effect |
|
Vasoconstriction |
Apoptosis |
||||
Figure 9-3. A simple signaling paradigm. The most conceptually simple lipid signaling paradigms involve ligand-induced activation receptor complex, the recruitment of signaling proteins, and the activation of enzymes that produce signaling lipids. These lipids either recruit proteins to the membrane from the hydrophilic compartments or bind to proteins within the membranes themselves. Lipid-protein interactions result in changes in e ector protein function, leading to the downstream activation of signaling components and a resultant biological response. TNFR, tumor necrosis factor receptor; GPCR, G- protein coupled receptor.
the activation of PKC both in vitro and as a component of signaling induced by the pleiotropic proinflammatory cytokine tumor necrosis factor-α (TNF-α). Additional studies found that TNF-α could induce ceramide production via SM hydrolysis and that SM-derived ceramide could also modify cell behavior. Thus the field of sphingolipid signaling was born. Once the connections between TNF-α signaling and ceramide were established, it was not long before this lipid was being studied in the context of apoptosis. Although many questions remain today, three main lines of evidence support the hypothesis that ceramide is a key mediator of programmed cell death.
3.2.1. Ceramide induces apoptosis
One of the first connections made between ceramide and apoptosis was the discovery that short-chain, cell-permeable analogs of ceramide (e.g., N-acetyl- sphingosine, or C2-ceramide) were able to induce apoptosis in leukemia cell lines. The closely related molecule C2-dihydroceramide, however, was unable to induce apoptosis, suggesting that the effect was highly specific to the molecular configuration of ceramide.
Several studies followed to establish the importance of ceramide’s 4–5 trans double bond for its proapoptotic effects. Short-chain ceramides (C2–8) induce apoptosis that involves activation of members of the mitogenactivated protein kinase (MAPK) family (e.g., p38-MAPK and c-Jun N-terminal kinase), although the dependence on these signaling pathways is controversial. Shortchain analogs also modulate the activities of protein phosphatases such as PP1 and PP2A that can function to de-phosphorylate prosurvival proteins such as Akt. In addition to its direct effects, C6-ceramide, and to a lesser extent C2-ceramide, can be metabolized by the salvage pathway to form long-chain ceramides. Moreover, C6-ceramide can serve as a substrate for the ceramide transfer protein CERT and thus may modify levels of endogenous ceramide.
Currently, many researchers use C2- and C6-ceramide applied exogenously to induce cell death, but several other forms of ceramide can also reproduce similar effects. In some cell types, the addition of the bacterial sphingomyelinase from Bacillus cereus is sufficient to produce ceramide and cause cell death. Long-chain ceramides (e.g., C16–ceramide) dissolved in appropriate solvent mixtures such as dodecane/ethanol are also able to promote apoptosis. Interest in the proapoptotic abilities of ceramide have led to the development of ceramide analogs and ceramide-containing liposomes for clinical use in the treatment of a variety of diseases.
3.2.2. Ceramide accumulates during programmed cell death
After stimulation of cells in culture with a deathpromoting ligand such as TNF-α or genotoxic stress such as doxorubicin, one or more SMases are activated, leading to the hydrolysis of SM and accumulation of ceramide (Figure 9-4). These events typically happen within the first 30 minutes after stimulation, and the activities of the SMases often return to basal levels within 1 hour (Figure 9-5). However, prolonged stimulation results in a second wave of ceramide production that, although less well characterized, often depends on de novo ceramide synthesis (e.g., CerS activation) (Figure 9-6). Both SMaseand de novo–mediated ceramide production have been described for a variety of diverse cell-death stimuli (Figure 9-7). Details about these two means of ceramide production, as well as their significance in cell death, are provided later in the chapter.
3.2.3. Inhibition of ceramide production alters cell death signaling
In many experimental models of apoptosis, deathinduced ceramide production can be inhibited using
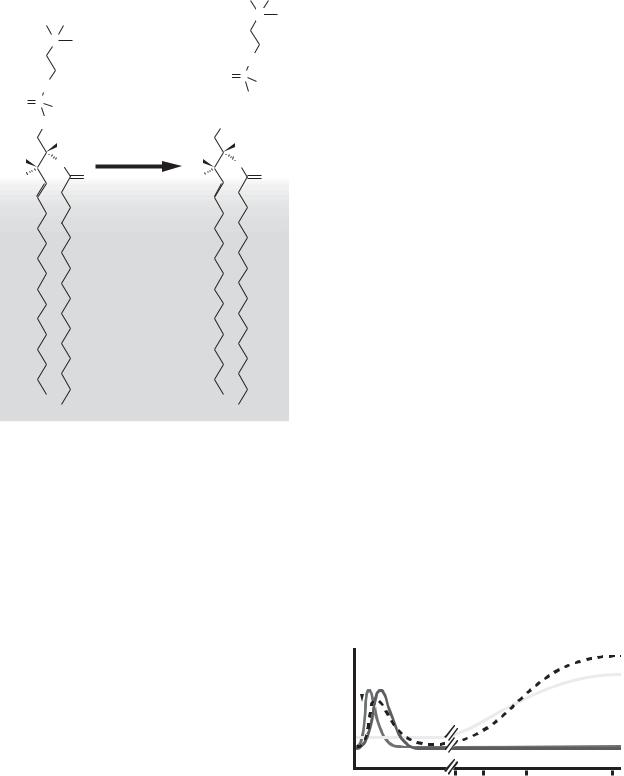
94 |
THOMAS D. MULLEN, RUSSELL W. JENKINS, LINA M. OBEID, AND YUSUF A. HANNUN |
|
|
|
+ |
|
|
|
|
N |
|
|
+ |
|
|
|
|
N |
|
|
|
|
|
|
O |
|
|
|
O |
P - |
|
|
O |
|
O |
|
|
|
O - |
||
O P |
O- |
+ |
||
|
||||
|
|
|
||
|
O |
O H |
|
|
|
H |
SMase |
H |
|
|
|
|||
H O |
N H |
H O |
N H |
|
H |
O |
H |
O |
Figure 9-4. Ceramide generation via SMase activation. Activation of SMases leads to hydrolysis of SM to form ceramide and phosphocholine.
pharmacological, genetic, or more recent RNA inter- ference-based approaches. In many cases, such inhibition impedes the cell death process. For example, activation of CD95 on lymphocytes leads to SMase activation, ceramide production, receptor clustering, and apoptosis. Cells deficient in a particular SMase (see Sections 4.2 and 4.3) fail to achieve the same responses and are protected from cell death. In other systems, inhibition of de novo ceramide synthesis using pharmacological inhibitors of SPT or CerS prevents apoptosis. As we discuss next, cell death signaling can occur via multiple ceramide-mediated pathways that differ in terms of time, subcellular localization, and involvement of particular enzymes of sphingolipid metabolism.
4. CERAMIDE MEDIATES APOPTOTIC CELL DEATH:
ROLE OF PARTICULAR ENZYME SYSTEMS
To illustrate the salient features of regulated sphingolipid metabolism and the ceramide-mediated death signaling, we discuss selected studies that highlight key features regarding the nature of sphingolipids as signaling molecules in cell death.
4.1. Ceramide is generated through SM hydrolysis
Hydrolysis of SM by SMases involves cleavage of the phosphodiester bond of SM releasing the phosphorylcholine head group to generate ceramide in a single, rapid step (Figure 9-4). By virtue of its capacity to generate ceramide acutely, the SMase/ceramide pathway is commonly studied in the context of acute cellular signaling. There are several mammalian SMases that are classified by pH optima for enzymatic activity and requirement for divalent cations. Of the putative mammalian sphingomyelinases that have been identified and cloned, acid sphingomyelinase (aSMase, SMPD1) and Mg2+-dependent neutral sphingomyelinase 2 (nSMase2, SMPD3) have been implicated in regulated SM hydrolysis in response to a range of stress stimuli. Although elevations in both nSMase2 and aSMase activity have been reported in response to apoptotic mediators, the involvement of nSMase2 in apoptosis has not been well defined. In contrast, there is abundant evidence linking the aSMase/ceramide pathway to apoptotic signaling.
aSMase catalyzes the cleavage of SM to ceramide at an optimum pH of 4.5 to 5.5, befitting its localization within the acidic endo-lysosomal compartment. Deficiency of aSMase results in Niemann-Pick disease (NPD), a lysosomal storage disorder characterized by multiple organ defects and, at the cellular level, by accumulation of SM within lysosomes. In the mid-1990s, as ceramide was gaining attention as a bioactive lipid, it was discovered that cells derived from NPD patients and aSMase knockout mice were resistant to stress-induced apoptosis in response to a variety of stimuli. Cells and
death stimulus
activationenzymerelative or accumulationceramide |
|
nSMase |
|
|
|
||
|
|
aSMase |
|
|
|
||
|
|
|
|
|
|
|
|
0 |
1 |
||
ceramide
de novo
2 6 12 24 time (hours)
Figure 9-5. Time dependence of ceramide accumulation in cell death. Besides a few exceptions, the time course of enzyme activation and ceramide accumulation appears to di er between each particular enzyme system. Activation of nSMase and/or aSMase occurs within minutes of stimulation, whereas de novo synthesis is increased after several hours.

CERAMIDE AND LIPID MEDIATORS IN APOPTOSIS |
95 |
CoA-SH
4.2. aSMase is activated after activation of
+extracellular receptors to promote apoptosis
|
|
|
|
|
|
|
|
|
|
|
OH |
TNF-α has established roles in the regulation and patho- |
||||||
|
|
CoA |
|
OH |
|
CerS |
|
|
|
|
physiology of inflammatory processes, but high levels |
|||||||
|
|
|
|
|
|
|
|
H |
||||||||||
|
S |
|
|
|
|
|
of TNF-α can induce cell death in some cell types. One |
|||||||||||
|
|
|
H |
|
|
|
|
|
||||||||||
|
|
O |
+ |
|
|
HO |
NH |
|||||||||||
|
|
HO |
+ |
|
|
|
|
of the seminal studies demonstrating an in vivo role |
||||||||||
O |
|
|
NH 3 |
|
|
H |
|
|
O |
|||||||||
|
|
H |
|
|
|
|
for aSMase in TNF-α/TNF receptor-mediated apoptosis |
|||||||||||
|
|
|
|
|
|
|||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
defined a role for aSMase in endotoxic shock syndrome. |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intraperitoneal administration of the lipopolysaccha- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ride (LPS; also known as endotoxin), a lipid from |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gram-negative bacteria, induces disseminated endothe- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
lial cell apoptosis. In response to LPS, loss of endothe- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
lial integrity causes damage to the lung, intestine, fat, |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
and thymus. Within these tissues, such damage is pre- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ceded by ceramide formation. Similarly, direct admin- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
istration of TNF-α is also capable of inducing ceramide |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
generation within several hours. Blocking TNF-α action |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
using an inhibitory peptide protected animals from LPS- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
induced ceramide generation and endothelial cell apop- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
tosis, supporting the notion that TNF-α is an interme- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
diary of LPS-induced signaling. Interestingly, aSMase |
||||
Figure 9-6. Ceramide generation via CerS activation. Ceramide can |
knockout mice are resistant to the effects of LPS despite |
|||||||||||||||||
a normal elevation in TNF-α, suggesting that aSMase is a |
||||||||||||||||||
accumulate when CerS are activated, leading to enhanced ceramide |
||||||||||||||||||
synthesis from dihydrosphingosine (via dihydroceramide, not pic- |
crucial mediator of the apoptotic response downstream |
|||||||||||||||||
tured) or sphingosine. |
|
|
|
|
|
|
|
|
of TNF-α elevation. Lastly, direct injection of TNF-α |
|||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
is capable of inducing endothelial apoptosis in wild- |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
type mice, whereas aSMase-deficient mice are markedly |
||||
tissues deficient in aSMase exhibited enhanced survival |
protected, proving that the aSMase/ceramide pathway |
|||||||||||||||||
in response to multiple inducers of cell death, whereas |
mediates LPS/TNF-α–induced ceramide generation and |
|||||||||||||||||
wild-type counterparts underwent apoptosis. Protection |
subsequent endothelial cell death. |
|
||||||||||||||||
from cell death in aSMase-deficient cells in response to |
The aSMase/ceramide pathway is also a crucial |
|||||||||||||||||
receptor-mediated and receptor-independent death sig- |
player in TNF-α–mediated liver disease. Osawa et al. |
|||||||||||||||||
nals was attributed to impaired ceramide generation, |
(2005) demonstrated that TNF-α–induced hepatocyte |
|||||||||||||||||
rather than accumulation of SM, sup- |
|
|
|
|
|
|
|
|
||||||||||
porting a positive role for aSMase- |
nSMase |
|
|
|
aSMase |
|||||||||||||
derived |
ceramide in |
the induction |
TcR |
S. aureus |
||||||||||||||
of stress-induced apoptosis. Further- |
|
|
|
|
|
|||||||||||||
|
|
|
|
CD40 |
hypoxia |
P. aeruginosa |
|
|||||||||||
more, many of |
these |
same |
death- |
|
|
|
|
|
||||||||||
|
|
|
H2O2 |
|
||||||||||||||
|
|
|
TNF-α |
Rhinovirus |
||||||||||||||
inducing stimuli |
have |
been |
shown |
|
|
NO |
|
|||||||||||
|
|
|
FasL |
cisplatin |
|
|||||||||||||
to induce |
relocalization of |
aSMase |
|
|
|
|
||||||||||||
|
|
|
daunorubicin |
|
||||||||||||||
|
|
|
doxorubicin |
paclitaxel |
|
|||||||||||||
from the endo-lysosomal compart- |
|
|
|
|
||||||||||||||
|
|
|
|
BcR X-linking |
IR |
actinomycin-D |
|
|||||||||||
ment to the outer leaflet of the plasma |
|
|
|
|
|
UV |
Isch/Rep |
|
||||||||||
membrane, |
a rich source of |
sphin- |
|
|
|
|
|
|
|
|||||||||
|
|
|
|
|
etoposide |
|
|
|||||||||||
gomyelin. This form of aSMase at the |
|
|
|
|
|
|
|
|||||||||||
|
|
|
|
cannabinoids |
|
|
||||||||||||
plasma membrane is considered cru- |
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||
cial for |
both receptor-mediated and |
|
|
|
|
De novo |
|
|
||||||||||
receptor-independent cell death sig- |
|
|
|
|
|
|
||||||||||||
|
|
|
|
|
|
|
|
|||||||||||
naling, |
although |
the mechanism of |
Figure 9-7. Multiple proapoptotic stimuli activate ceramide production and with di erent |
|||||||||||||||
relocalization and the precise molecu- |
kinetics. Depending on the stimulus, ceramide accumulation has been attributed to the acti- |
|||||||||||||||||
vation of several enzyme systems, most notably activation of nSMase, aSMase, or the de novo |
||||||||||||||||||
lar identity of this “activated” form of |
||||||||||||||||||
pathway. NO, nitric oxide; TcR, T-cell receptor; BcR X-linking, B-cell receptor cross-linking; UV, |
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|||||||||
aSMase remain poorly defined. |
ultraviolet light; Isch/Rep, ischemia/reperfusion injury |
|
||||||||||||||||
