
- •Contents
- •Vocabulary
- •Elements and compounds
- •1.6. Read the following text and say if it is true that interatomic distance is fixed in all states of a metal. Read again to answer the questions after it.
- •Vocabulary
- •Three states of matter
- •1.10. Learn to read the following measurements.
- •1.11. Read out the numbers.
- •1.12. A) Compare the spanners. Make sentences.
- •At the Descriptive Geometry Class
- •Vocabulary
- •Characteristic Features of Some Elements
- •Vocabulary Test
- •Grammar Test
- •Vocabulary
- •Materials science in the past and present
- •2.9. Form nouns from the following words:
- •Vocabulary
- •Engineering materials and their properties (Part I)
- •2.12. Reread the text and rewrite the following according to the model, replacing the words in italics with an expression from the text which has a similar meaning.
- •2.16. Read and translate the following text. Talk about the properties of engineering materials in your own words.
- •Vocabulary
- •Engineering materials and their properties (Part II)
- •Vocabulary Test
- •Grammar Test
- •Unit 3. Metals: properties, classification and crystal structure
- •Read the list of words below and choose the ones related to science of materials:
- •Vocabulary
- •Metals, alloys and their uses
- •3.4. Reading comprehension. Read the text Availability, Properties and Classification of Metals and for questions 1–5 (after the text) choose the best answers from a–d.
- •Vocabulary
- •Availability, properties and classification of metals
- •3.5. Use the questions and talk giving the main ideas of the text above.
- •Vocabulary
- •Metallic crystal structure
- •Vocabulary Test
- •Grammar Test
- •Unit 4. Engineering materials. Iron and ferrous metals
- •4.2. Read the text Iron and Its Properties. Answer the following questions. What new have you learnt from the text?
- •Vocabulary
- •Iron and its properties
- •Vocabulary
- •4.4. Connect the two matching parts of the sentences related to the blast furnace operation.
- •Vocabulary
- •Ferrous metals
- •From the history of steelmaking
- •Alloy steels
- •Grammar and Vocabulary Questionnaire
- •Structural steels for shipbuilding
- •Vocabulary Test
- •Grammar Test
- •4.17. Just for fun.
- •4.18. Read the text and agree and disagree with the statements after it.
- •4.19. In the above text, find the English equivalents for the following words and word combinations:
- •4.20. Read the text and write a list of titanium and its alloys qualities that make titanium different from other metals. A wonder metal
- •Long-term corrosion protection for hulls and water jets
- •Nonmetallic materials
- •4.24. What kinds of non-metal things do people use at home and at work in the office? Entitle the text below. Compare metals and non-metals as structural materials.
- •Unit 5. Materials technology
- •Vocabulary
- •Processing and heat treatment of metals
- •Visit to a Plant
- •Hardening plain carbon steel
- •Vocabulary
- •Welding processes
- •Gas welding
- •Hard to define
- •Nanotechnology
- •Larger to smaller: materials perspective
- •References
- •Appendix Summary tips Аннотирование и реферирование
- •Аннотация и реферат
- •Структура реферата
- •Этапы реферирования и аннотирования
- •Некоторые рекомендации по составлению аннотации и реферата
3.5. Use the questions and talk giving the main ideas of the text above.
1. What does the availability of metals depend on?
2. What does metallurgy deal with?
3. What is the broader usage of the word “metal”?
4. What properties are generally assigned to metals?
5. What non-metals are rather good conductors of heat and electricity?
6. What is the commercial classification of metals?
7. Ferrous metals are used in most metallic products, aren’t they?
3.6. Choose either an adjective or an adverb to complete each of the sentences below.
Alloys are (general, generally) prepared by mixing molten metals.
Pure iron is a (softly, soft) metal.
Platinum has (exceptional, exceptionally) resistance to corrosion.
Iron is (easy, easily) to extract from iron ores.
Rolled gold consists of a (thin, thinly) layer of gold alloy.
Radium is an extremely (rare, rarely) metal, it is also (high, highly) radioactive.
Magnesium is known as a metal, which burns (bright, brightly).
3.7. Read and translate the following text.
Vocabulary
grain n |
— |
зерно, кристалл |
behaviour n |
— |
поведение, характеристики |
packing n |
— |
упаковка (атомов) |
close-packed pp |
— |
плотноупакованный |
lattice n |
— |
решетка |
face n |
— |
грань |
face-centred cubic (fcc)
|
— |
гранецентрированный кубический (ГЦК) |
hexagonal close-packed (hcp)
|
— |
гексагональный плотноупакованный (ГП) |
body-centred cubic (bcс)
|
— |
объемноцентрированный кубический (ОЦК) |
heat treatment |
— |
термообработка |
undergo v |
— |
подвергаться, испытывать |
phase change |
— |
фазовое превращение |
nucleation n |
— |
зарождение центров кристаллизации |
orientation n |
— |
ориентация (зерен) |
grain boundaries |
— |
границы зерен |
strengthen v |
— |
упрочнять |
weaken v |
— |
ослаблять |
Metallic crystal structure
All chemical elements, including the metals, are composed of atoms. It is important to know how the atoms are arranged in a grain of metal to explain the behaviour of metals.
Atoms can be arranged in many different ways in crystalline solids, but in metals the packing is one of three simple forms. In the most ductile metals, atoms are arranged in a close-packed manner. In Fig. 1 we can see a crystal with cubic symmetry that could be visualized as an assembly of cubes with atoms at the corners and at the centre of each face (known as face-centred cubic, or fcc). Metals with the fcc structure include aluminium, copper, nickel, gamma iron, gold and silver. Fig. 2 shows a crystal with hexagonal symmetry (called hexagonal close-packed, or hcp). Examples of metals with the hcp type of structure are magnesium, zinc, alpha titanium.

The third common crystal structure in metals can be visualized as an assembly of cubes with atoms at the corners and an atom in the centre of each cube (Fig. 3). This is known as body-centred cubic, or bcc. Examples of metals with the bcc structure are alpha iron, chromium and beta titanium.
Some metals, such as titanium and iron, show different crystal structures at different temperatures. The lowest-temperature structure is called alpha (α), and higher-temperature structures – beta (β), gamma (γ) and delta (δ). This allotropy, or transformation from one structure to another with changing temperature, leads to the changes in properties that can come from heat treatment.
When a metal undergoes a phase change from liquid to solid or from one crystal structure to another, the transformation begins with the nucleation and growth of many small crystals of the new phase. All these crystals or grains have the same structure but different orientations, so that, when they finally grow together, boundaries form between the grains. These boundaries play an important role in determining the properties of metals. At room temperature they strengthen the metal without reducing its ductility, but at high temperatures they often weaken the structure and lead to early failure. They can be the site of localized corrosion which also leads to failure.
3.8. According to the text, agree or disagree with the following statements. Use the following ways of agreeing or disagreeing. Explain your view.
I fully / completely / entirely agree. I agree up to the point, but … I partly agree because ... |
I don't really agree. I don't agree at all.
|
Metals belong to the class of substances which are crystalline.
Most common metals have either face-centred cubic, body-centred cubic or hexagonal atomic lattice.
In zinc, magnesium, alpha titanium the atoms are arranged in a body-centred cubic pattern.
Iron atoms do not change their lattice at different temperatures.
Metal properties can be changed with heat treatment.
At high temperatures grain boundaries often strengthen the metal structure.
3.9. Read the text about D. Chernov and talk about his scientific interests and achievements.
D mitry
Chernov (1839 – 1921), a great Russian metallurgist and a founder
of modern physical metallurgy, was born in St. Petersburg. He
graduated from St. Petersburg Technological Institute and began his
practice at one of the mechanical plants. There, he got a chance to
study mechanical and metallurgical processes. Later on, he worked as
a lecturer at St. Petersburg University. But then he returned to his
work at the metallurgical plant.
mitry
Chernov (1839 – 1921), a great Russian metallurgist and a founder
of modern physical metallurgy, was born in St. Petersburg. He
graduated from St. Petersburg Technological Institute and began his
practice at one of the mechanical plants. There, he got a chance to
study mechanical and metallurgical processes. Later on, he worked as
a lecturer at St. Petersburg University. But then he returned to his
work at the metallurgical plant.
Chernov
paid his attention to metal forging, studied thermal processes, low-
and high-tempering of steel. The scientist showed that the structure
of steel changes by heating and discovered structural transformation
temperatures i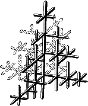 n
steel, so-called Chernov’s points.
n
steel, so-called Chernov’s points.
Chernov published scientific works “On Influence of Mechanical and Thermal Treatment on the Properties of Steel”, “Analysis of the structure of Cast Steel Ingots”, etc. He developed a theory of metal crystallization and metallography (in the left-hand picture you can see a schematic dendritic crystal according to D. Chernov).
3.10. a) Read and entitle the text. b) Ask questions on each paragraph of the text and let your partner answer them. c) Write a summary of the text. For tips on writing a summary, see Appendix.
In ancient times people learned to use certain materials which were easy to obtain, such as wood, skins, stone and eventually a few metals. Man’s going from stone to metal was long and complicated. The first metal to be used by man was copper. The first records about this metal go back to 6500–5700 BC. Gold, silver and copper existing free in nature were employed principally in the manufacture of jewelry, dishes and coins.
The art of blending metals was gradually developed. Some alloys obtained in this way were stronger, harder or tougher than the metals themselves. They discovered that tin mixed with copper gave a harder substance. So there came into being the alloy that we call bronze. The Bronze Age began in Egypt around 3000 BC and in Europe some 500 or 1000 years later.
The following stage was the Iron Age. Since almost no iron exists free in nature, it undoubtedly came into general use later than the above metals. Producing pure iron and its alloys was made possible with the accumulated experience in smelting copper, bronze, gold and other metals and alloys. Mastering iron production gave a powerful incentive to the development of productive forces and technical progress.
In ancient times people knew eight metals. By the end of the eighteenth century their number had increased by twenty. Nowadays about 80 metals are produced, and they are the most abundant of materials to supply man’s needs in manufacturing advanced tools, machines, devices and other metal objects.
Notes
records pl n – упоминание, записи blending – смешение, составление шихты |
mastering – освоение incentive n – побуждение, стимул
|
3.11. Translate the sentences. Remember the verbs with their prepositions.
1. This question was agreed upon after a prolonged discussion. 2. The method is referred to in his article. 3. His experience can be relied upon. 4. Our arguments were listened to with great attention. 5. This strange fact cannot be easily accounted for. 6. In spite of all their efforts the decision has not been arrived at. 7. This scientist had been always spoken about with admiration. 8. The research results will be commented on at the conference.
3.12. In the following sentences since means так как, с тех пор, как, с. Translate the sentences.
1. I have been studying here since last year.
2. Since he started that diet, he’s lost over 20 lbs in weight.
3. Since you cannot answer, we should ask someone else.
4. Since you have finished your work, you may go home.
3.13. For fun and profit. Read the joke and say what rules of writing friendly and business letters you know.
A Letter to a Friend
During vacation time a student made up his mind to write a letter to his friend. He sat down at his writing-table and wrote:
“Dear Bill!” He thought a bit and continued: “I’m writing to you because I have nothing to do.” After that he thought a great deal and added at last: “I’m afraid I must stop writing to you because I have nothing to say. Yours truly, Tom Brown.”
3.14. Joining ideas. To add information, we can use: furthermore, moreover, similarly, what is more, besides, in addition, also, both … and …, etc. To express contrasting ideas, we can use: however, nevertheless, although/though, on the other hand, whereas, while, in spite of, despite, in contrast, etc.
Examples: 1) Self-defence classes can help you to protect yourself. Furthermore, they keep you fit.
2) Travelling by aeroplane is fast, however, it is expensive.
Read the sentences and choose the right word.
1. Being a doctor is very demanding. Furthermore/However, it is a job in which there is no room for mistakes. 2) Exercising helps us to keep fit. Nevertheless/Moreover, it can be lots of fun. 3) Driving to work can be convenient. On the other hand/Similarly, finding a place to park can be a problem. 4. Living in a foreign country can be very difficult. In contrast/Furthermore, one can often feel lonely and homesick. 5) Going on holiday is a great way to relax. Similarly/ Nevertheless, taking short trips at the weekend can also be enjoyable. 6) Cities are noisy. Also/In contrast, the countryside is quiet. 7) Living on your own teaches you to be independent. Also/However, it helps you to become more responsible.
b) Join the following sentences.
1. Dogs are domesticated. Wolves are wild.
2. The jaguar is fast. It is also beautiful.
3. Elephants are very intelligent. They are social animals.
4. They have been cleaning up the beach. It is still dirty.
5. He doesn’t approve of killing animals. He likes eating meat.
6. The hole in the ozone layer is growing. Not enough is being done to control industrial pollution.
7. Some people want hunting to be banned. It is still a popular sport.
3.15. Test yourself. Choose the best answers from a–d for the questions in the tests.
