9.4 RET between like molecules. Excitation energy migration in assemblies of chromophores 265
9.4.2
RET in assemblies of like chromophores
For assemblies of like chromophores in three dimensions in an infinite volume, many theories have provided various expressions of the survival probability GsðtÞ. Only one of them will be given here, owing to its simplicity and good accuracy. Huber (1981) obtained the following relationship:
|
|
|
|
|
|
|
|
|
|
# |
|
Gs t |
rðtÞ |
|
exp |
2g0 |
t |
1=2 |
9:39 |
ð Þ ¼ |
r0 |
¼ |
|
" |
|
t0 |
|
ð Þ |
where t0 is the intrinsic excited-state lifetime and g0 is given by |
|
g0 ¼ p2 |
¼ |
2p2 C |
3 pR30 |
|
|
|
ð9:40Þ |
|
g |
|
pp |
|
4 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
where C is the concentration of molecules (number of molecules/A˚ 3), and R0 is the critical Fo¨rster radius (with the assumption of orientation-averaged interaction).
Investigations of excitation energy transport among chromophores are of major interest in understanding how nature harvests sunlight. In fact, excitation energy migrates among chlorophyll molecules within the antennae pigments and is eventually trapped in the reaction center, where it is converted into chemical energy. Considerable e ort has been devoted to the understanding of energy migration in photosynthetic units with the help of fluorescence techniques (Van Grondelle et al., 1996; Pullerits and Sundstro¨m, 1996).
Excitation energy transport has also been studied in artificial systems such as polymers having chromophores substituted at intervals along the chains (Guillet, 1985; Webber, 1990), polynuclear complexes (Balzani, 1992) and multi-chromo- phoric cyclodextrins (Berberan-Santos et al., 1999). These studies are of interest not only in providing a better understanding of the light-harvesting step of photosynthesis, but also in designing photomolecular devices for conversion of light (Balzani and Scandola, 1990).
9.4.3
Lack of energy transfer upon excitation at the red-edge of the absorption spectrum (Weber’s red-edge e ect)
Section 3.5.1 described the various e ects observed upon excitation at the red-edge of the absorption spectrum. In particular, a lack of energy transfer was first observed by G. Weber (1960) (and is called Weber’s e ect for this reason). This e ect can be explained in terms of inhomogeneous broadening of spectra. In a rigid polar solution of fluorophores that are close enough to undergo non-radiative en-
266 9 Resonance energy transfer and its applications
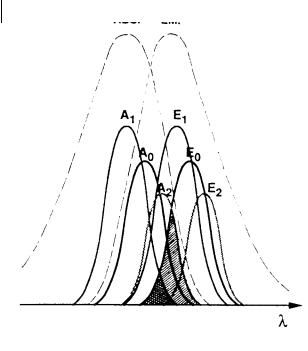
Fig. 9.4. Inhomogeneous spectral broadening responsible for directed energy transfer. The spectral overlap between the emission spectrum E0 of an excited species (whose absorption spectrum is A0) and the absorption spectrum A2 of a solvate absorbing at higher
wavelengths (cross-hatched area þ double cross-hatched area) is larger than the spectral overlap with the absorption spectrum A1 of a solvate absorbing at lower wavelengths (double cross-hatched area).
ergy transfer, the probability of transfer from a chromophore in a given configurational state to a chromophore whose configurational state corresponds to a lower electronic transition frequency is greater than the probability of transfer to a chromophore with a higher electronic transition frequency. This is because of the smaller overlap in the latter case between the donor fluorescence and acceptor absorption spectra, as illustrated in Figure 9.4.
In both mechanisms of transfer by dipole–dipole interaction or exchange interaction, the e ciency of transfer is indeed related to the spectral overlap. Therefore, the transfer from ‘blue’ solvates to ‘red’ ones is more probable than the reverse transfer. As back transfer has a lower probability, we can speak of directed nonradiative energy transfer. This explains the lack of energy transfer upon red-edge excitation: as the excitation wavelength increases beyond the absorption maximum, energy hopping from the directly excited chromophore becomes less and less probable because the proportion of ‘blue’ partners to which transfer is weak or impossible drastically increases. In other words, energy hopping is spectrally selective. Such a directed energy hopping may be of major importance in energy transport from the antenna to the reaction center in photosynthetic membranes. An example of a very pronounced red-edge e ect is given in Box 9.3.
9.4 RET between like molecules. Excitation energy migration in assemblies of chromophores 267
Box 9.3 Red-edge e ect in multi-chromophoric cyclodextrinsa)
A b-cyclodextrin bearing seven 2-naphthoyloxy chromophores, CD7(6), is a good model for studying the e ect of the excitation wavelength on energy hopping among chromophores in well-defined positions, as in photosynthetic antennae.
Fig. B9.3.1. A: absorption spectrum of the multi-chromophoric cyclodextrin CD7(6) and variations in the emission maximum as a function of the excitation wavelength (broken line).
B: excitation polarization spectra of the model compound NAEt and CD7(6). Solvent: mixture (9:1 v/v) of propylene glycol and 1,4-dioxane at 200 K (adapted from Berberan-Santos et al.a)).

268 9 Resonance energy transfer and its applications
Absorption spectra, emission spectra and excitation polarization spectra were recorded in a propylene glycol–dioxane glass at 200 K. Comparison was made with the reference chromophore 2-ethylnaphthoate (NAEt).
Because the rotational motions of the chromophores are frozen in the rigid glass, the depolarization e ect observed with CD7(6) as compared with NAEt (see Figure B9.3.1) is solely caused by non-radiative electronic energy transfer, i.e. energy hopping between chromophores. In fact, an emitting chromophore that is indirectly excited by energy transfer is likely to have its emission transition moment oriented di erently from that of the directly excited one.
When increasing the excitation wavelength beyond 335 nm, the emission anisotropy drastically increases, thus indicating a gradual decrease in energy transfer e ciency. At the extreme red-edge (A350 nm) there is a complete lack of energy transfer (Weber’s e ect). It is also noticed that the increase in emission anisotropy at wavelengths ranging from 320 to 328 nm (longwave edge of the second vibronic band) is significantly steeper for CD7(6) than for NAEt. Furthermore, the maximum of emission anisotropy around 300 nm (i.e. at the longwave edge of the second electronic band) is located at higher wavelengths for CD7(6) than for NAEt. We can thus speak of a vibronic red-edge e ect.
Figure B9.3.1 shows the parallelism between the increase in emission spectrum displacement and fluorescence anisotropy observed for the red-edge of most vibronic bands and especially for the 0–0 one. It can be interpreted in terms of inhomogeous spectral broadening due to solvation heterogeneity. The decrease in energy transfer that is observed upon red-edge excitation is evidence that energy hopping is not chaotic but directed toward lower energy chromophores, as in photosynthetic antennae.
a)Berberan-Santos M. N., Pouget J., Valeur B., Canceill J., Jullien L. and Lehn J.-M. (1993) J. Phys. Chem. 97, 11376–9.
9.5
Overview of qualitative and quantitative applications of RET
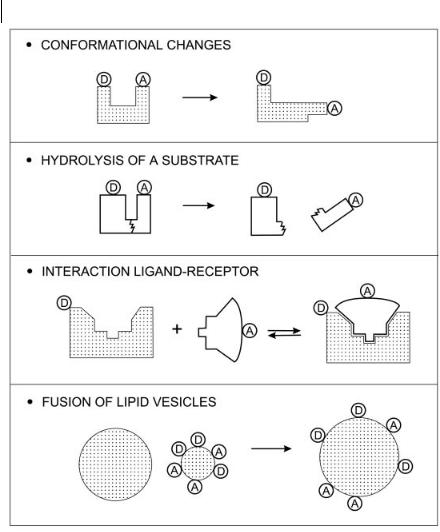
In addition to the determination of distances at a supramolecular level, RET can be used to demonstrate the mutual approach of a donor and an acceptor at a supramolecular level as a result of aggregation, association, conformational changes, etc. The donor and acceptor molecules are generally covalently linked to molecular, macromolecular or supramolecular species that move toward each other or move away. From the variations in transfer e ciency, information on the spatial relation between donor and acceptors can thus be obtained. Because of its simplicity, the steady-state RET-based method has been used in many diverse situations as shown below5).
5)Leading references can be found in the reviews by Cheung (1991), Clegg (1996), Valeur (1993), Webber (1990), Wu and Brand
(1994) and the books by Guillet (1985), Van der Meer et al. (1994).
9.5 Overview of qualitative and quantitative applications of RET 269
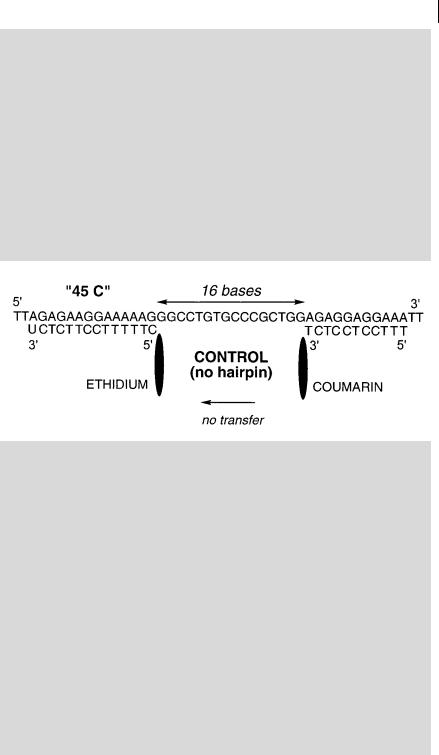
Box 9.4 Formation of hairpin structures as studied by RETa)
Single-stranded DNA or RNA may adopt hairpin structures in which the distance between two sequences is much shorter than in the absence of hairpin. Figure B9.4.1 shows two synthetic targets, both containing 45 nucleotides, but only the first one is able to form a hairpin via a loop of four thymines. The second one is used as a control. Both contain the complementary sequences for ethidium–13-mer and 11-mer–coumarin separated by the same number of bases. The e ciency of energy transfer from coumarin to ethidium is close to zero for the control, whereas it is about 25% in the hairpin structure. This value is low but the spatial conformation of this particular three-way junction is only partially known, and the transfer e ciency depends on the relative orientation and/or distance between coumarin and ethidium.
Fig. B9.4.1. Sequences of the 45-mer containing a hairpin structure and the control 45-mer (redrawn from Mergny et al.a)).
a)Mergny J. L. et al. (1994) Nucl. Acids Research 22, 920–8.
270 9 Resonance energy transfer and its applications
Fig. 9.5. Some applications of RET.
Chemical sciences
. Scintillators
.Polymers: interpenetration of polymer chains, phase separation, compatibility between polymers, interdi usion of latex particles, interface thickness in blends
of polymers, light-harvesting polymers, etc.
. Supramolecular systems: molecular devices, artificial photosynthesis, antenna e ect, etc.
. Restricted media and fractals. Morphology of porous solids. Determination of dimensionality.
. Chemical sensors
9.6 Bibliography 271
Life sciences
. Ligand–receptor interactions
. Conformational changes of biomolecules
. Proteins: in vivo protein–protein interactions, protein folding kinetics, protein subunit exchange, enzyme activity assay, etc.
. Membranes and models: membrane organization (e.g. membrane domains, lipid distribution, peptide association, lipid order in vesicles, membrane fusion assays, etc.)
.Nucleic acid structures and sequences: primary and secondary structure of DNA fragments, translocation of genes between two chromosomes, detection of nucleic acid hybridization, formation of hairpin structures (see Box 9.4), interaction with drugs, DNA triple helix, DNA–protein interaction, automated DNA
sequencing, etc.
. Nucleic acid–protein interaction
. Immunoassays
. Biosensors
Some examples of these applications are shown in Figure 9.5.
9.6
Bibliography
Andrews D. L. and Demidov A. A. (Eds)
(1999) Resonance Energy Transfer, Wiley & Sons, New York.
Baumann J. and Fayer M. D. (1986) Excitation Energy Transfer in Disordered Two-Dimensional and Anisotropic ThreeDimensional Systems: E ects of Spatial Geometry on Time-Resolved Observables, J. Chem. Phys. 85, 4087–4107.
Balzani V. and Scandola F. (1990)
Supramolecular Photochemistry, Horwood, New York.
Balzani V., Campagna S., Denti G. and Serroni S. (1992) Supramolecular Photochemistry: Antenna E ect in Polynuclear Metal Complexes, in: Kochanski E. (Ed.), Photoprocesses in Transition Metal Complexes, Biosystems and Other Molecules. Experiment and Theory, Kluwer Academic Publishers, Dordrecht, p. 233.
Berberan-Santos M. N. and Valeur B.
(1991) Fluorescence Polarization by Electronic Energy Transfer in Donor–
Acceptor Pairs of Like and Unlike Molecules, J. Chem. Phys. 95, 8048–8055.
Berberan-Santos M. N., Choppinet P., Fedorov A., Jullien L., Valeur B. (1999)
Multichromophoric Cyclodextrins. 6. Investigation of Excitation Energy Hopping by Monte-Carlo Simulations and TimeResolved Fluorescence Anisotropy, J. Am. Chem. Soc. 121, 2526–2533.
Berlman I. D. (1973) Energy Transfer Parameters of Aromatic Compounds, Academic Press, New York.
Butler P. R. and Pilling M. J. (1979) The Breakdown of Fo¨rster Kinetics in Low Viscosity Liquids. An Approximate Analytical Form for the Time-Dependent Rate Constant Chem. Phys. 41, 239–243.
Cheung H. C. (1991) Resonance Energy Transfer, in: Lakowicz, J. R. (Ed.), Topics in Fluorescence Spectroscopy, Vol. 2, Principles, Plenum Press, New York, pp. 127–176.
Clegg R. M. (1996) Fluorescence Resonance Energy Transfer, in: Wang X. F. and Herman B. (Eds), Fluorescence Imaging
Molecular Fluorescence: Principles and Applications. Bernard Valeur
> 2001 Wiley-VCH Verlag GmbH
ISBNs: 3-527-29919-X (Hardcover); 3-527-60024-8 (Electronic)
273
10
Fluorescent molecular sensors of ions and molecules
La part de l’imagination dans le travail scientifique est la meˆme que dans le travail du peintre ou de l’e´vrivain. Elle consiste a` de´couper le re´el, et a` recombiner les morceaux pour cre´er quelque chose de neuf.
[The part played by the imagination in scientific work is the same as in the work of the painter or the writer. It consists of cutting up reality, and recombining the pieces to create something new.]
Francois Jacob, 1981
10.1
Fundamental aspects
The design of fluorescent sensors is of major importance because of the high demand in analytical chemistry, clinical biochemistry, medicine, the environment, etc. Numerous chemical and biochemical analytes can be detected by fluorescence methods: cations (Hþ, Liþ, Naþ, Kþ, Ca2þ, Mg2þ, Zn2þ, Pb2þ, Al3þ, Cd2þ, etc.), anions (halide ions, citrates, carboxylates, phosphates, ATP, etc.), neutral molecules (sugars, e.g. glucose, etc.) and gases (O2, CO2, NO, etc.). There is already a wide choice of fluorescent molecular sensors for particular applications and many of them are commercially available. However, there is still a need for sensors with improved selectivity and minimum perturbation of the microenvironment to be probed. Moreover, there is the potential for progress in the development of fluorescent sensors for biochemical analytes (amino acids, coenzymes, carbohydrates, nucleosides, nucleotides, etc.).
The success of fluorescent sensors can be explained by the distinct advantages o ered by fluorescence detection in terms of sensitivity, selectivity, response time, local observation (e.g. by fluorescence imaging spectroscopy). Moreover, remote sensing is possible by using optical fibers. The great improvement in the sensitivity
274 10 Fluorescent molecular sensors of ions and molecules
and the spatial or temporal resolution of instruments is also a factor in their success.
Many terms are used in the field of fluorescence sensing: fluorescent sensors, fluorosensors, fluorescent chemosensors, fluorescent molecular sensors, luminescent sensor molecules, luminescent sensors, fluorescent biosensors, fluorescent optical sensors, etc. It is important to make a clear distinction between the analyteresponsive (supra)molecular moiety, involving a fluorophore that signals the presence of an analyte by changes in its fluorescence characteristics, and the complete optical sensing device, i.e. the light source, the analyte-responsive (supra)molecular moiety properly immobilized (e.g. in plastified polymers, sol–gel matrices, etc.), the optical system (involving an optical fiber or not) and the light detector (photomultiplier or photodiode) connected to appropriate electronics for displaying the signal. In principle, a fluorescent sensor is the complete device, but in many papers, authors assign the term fluorescent sensor to the fluorescent analyte-responsive (supra)- molecular moiety as well. In order to avoid confusion, it is recommended in the latter case to use the term fluorescent molecular sensor.
Another distinction should be made (independently of the fluorescence aspects) between chemical sensors (also called chemosensors) and biosensors. In the former, the analyte-responsive moiety is of abiotic origin, whereas it is a biological macromolecule (e.g. protein) in the latter.
In fluorescent molecular sensors, the fluorophore is the signaling species, i.e. it acts as a signal transducer that converts the information (presence of an analyte) into an optical signal expressed as the changes in the photophysical characteristics of the fluorophore. In contrast, in an electrochemical sensor, the information is converted into an electrical signal.
The present chapter is restricted to fluorescent molecular sensors, for which three classes can be distinguished (Figure 10.1):
.Class 1: fluorophores that undergo quenching upon collision with an analyte (e.g. O , Cl ).
.Class 2: fluorophores that can reversibly bind an analyte. If the analyte is a proton, the term fluorescent pH indicator is often used. If the analyte is an ion, the term fluorescent chelating agent is appropriate. Fluorescence can be either
quenched upon binding (CEQ type: Chelation Enhancement of Quenching), or enhanced (CEF type: Chelation Enhancement of Fluorescence). In the latter case,2
the compound is said to be fluorogenic [e.g. 8-hydroxyquinoline (oxine)].
.Class 3: fluorophores linked, via a spacer or not, to a receptor. The design of such sensors, which are based on molecule or ion recognition by a receptor, requires special care in order to fulfil the criteria of a nity and selectivity. These aspects are relevant to the field of supramolecular chemistry. The changes in photophysical properties of the fluorophore upon interaction with the bound analyte are due to the perturbation by the latter of photoinduced processes such as electron transfer, charge transfer, energy transfer, excimer or exciplex formation or disappearance, etc. These aspects are relevant to the field of photophysics. In the case of ion recognition, the receptor is called an ionophore, and the whole molecular sensor is