
- •Астраханский государственный
- •Part 1. Знакомство
- •Let me introduce myself and my family
- •Vocabulary
- •About myself
- •Vocabulary.
- •Vladimir Vladimirovich Putin – the head of the rf government
- •Vocabulary
- •Тексты для самостоятельной работы студентов:
- •1. Accommodations and catering
- •2. Tourist attractions and entertainment
- •Тексты для самостоятельной работы студентов
- •Exercise 1
- •Freddie laker
- •Cesar manrique
- •Around the world in 222 days
- •Part 3. У врача. Медицинское обслуживание. Text 1
- •The laws of health
- •At the doctor`s
- •Text 1a
- •At the dentist`s
- •Medical assistance
- •The Doctor Arrival
- •In the Sick-Bay.
- •At the Hospital
- •The Doctor`s Advice
- •Тексты для самостоятельной работы студентов
- •Part 4. Моя страна. Мой город. Достопримечательности.
- •Vocabulary
- •Astrakhan
- •Vocabulary
- •Reading comprehension Text 1 Moscow
- •Text 2 The City of Astrakhan: history and present time
- •Text 3 The land of blooming lotus
- •Тексты для самостоятельной работы студентов:
- •Part 5. Страны изучаемого языка (Великобритания, сша, Австралия, Новая Зеландия):географические, политические и культурные аспекты. Canada
- •New Zealand
- •Australia
- •Great Britain
- •The usa
- •Washington, d.C.
- •Canberra
- •Wellington
- •Тексты для самостоятельной работы студентов
- •Part 6. Наш университет. Высшее образование в России.
- •Vocabulary
- •Moscow state lomonosov university
- •Тексты для самостоятельной работы студентов
- •Part 7. Высшее образование в стране изучаемого языка. Ведущие мировые университеты. Higher education in Great Britain
- •Тексты для самостоятельной работы студентов text I. Read the text to yourself and suggest a title.
- •Text IV. Stanford University
- •Part 8. Покупки. В магазине. Shopping
- •The Big Stores of London
- •Shopping phrases
- •Тексты для самостоятельной работы
- •Part 9. Война и мир. Угроза терроризма. World at war
- •Terrorism
- •Тексты для самостоятельной работы студентов
- •21St Century Terrorism
- •The eu fights against the scourge of terrorism
- •Part 10. Страны третьего мира. Проблемы миграции.
- •Тексты для самостоятельной работы студентов Text 1
- •List of emerging and developing economies
- •Developing countries not listed by imf
- •Industrialization
- •Part 11. Информатизация общества
- •Informatization
- •Origin of the term
- •Social impact of informatization
- •Informatization in economic systems
- •Globalization and informatization
- •Globalization
- •Definitions
- •Information technology
- •Information Age
- •The Internet
- •Progression
- •Тексты для самостоятельной работы студентов The Internet
- •Information
- •Communication in our life
- •Social impact of the Internet
- •What is Science?
- •Technology
- •Science, engineering and technology
- •Word Bingo
- •Alfred nobel - a man of contrasts
- •Alexander graham bell
- •3. What brought Einstein more joy than anything else?
- •4. By what illustration did Einstein explain his Theory of Relativity?
- •5. What two rules of conduct did Einstein have?
- •Part 13 Современные достижения науки. Перспективы развития науки.
- •Text 2. What is Nanotechnology?
- •Text 3. Collider
- •Text 4. Silicon Valley
- •Text 5. Small is beautiful
- •5____________________________________________________________
- •Text 6. Big is the Best
- •Тексты для самостоятельной работы студентов History of nanotechnology
- •Nanomaterials
- •Molecular nanotechnology
- •Collider design
- •Where have I heard that name before?
- •Part 14. Выдающиеся учёные прошлого Albert Einstein
- •Vocabulary of the text
- •Isaac Newton
- •Vocabulary of the text
- •Nicolaus Copernicus
- •Vocabulary of the text
- •Incoherency – несвязность, бессвязность, непоследовательность
- •Lomonosov, Mikhail Vasilyevich
- •Vocabulary of the text
- •Dmitriy Ivanovich Mendeleev
- •Vocabulary of the text
- •Influenza - грипп
- •Valence – валентность
- •Тексты для самостоятельной работы
- •Destroying forests
- •1 Damages
- •Language work
- •Language work
- •How the greenhouse effect works
- •Global warming
- •Тексты для самостоятельной работы студентов:
- •Atoms, molecules and compounds
- •Chemical reactions and chemical bonds.
- •Organic compounds and life
- •Carbohydrates
Exercise 6. Read the text.
Chemical reactions and chemical bonds.
When two or more atoms react to form compounds, chemical bonds are formed by
the attraction, sharing, or transfer to outer electrons from one atom to other. Such bonds between atoms can be broken, the atoms rearranged, and new bonds formed. A chemical reaction involves the making and breaking of chemical bonds. Chemical reactions are always occurring in living organisms. Living organisms are sometimes compared to chemical factories, bat the number and complexity of chemical reactions that occur in living organisms are vastly greater than in factory.
In chemical reactions, substances interact and form new bonds and new substances. These events are important in a cell for two reasons.
First, chemical reactions are the only way to form new molecules that the cell requires for such things as growth and tissue maintenance.
Second, the making and bracing of bonds involves changes in energy. As a result of chemical reactions in a cell, energy may be stored, used
to do work, or released. When atoms interact, they can form several types of chemical bonds. One type forms when electrons are transferred from one atom to another. This type of chemical bond occur in many substances, including table salt, also known as sodium chloride (NaCl). As the latter name suggests, table salt is made of two elements, sodium (Na) and chlorine(Cl). When atoms of these two elements react, an electron passed from a sodium atom to a chlorine atom. The resulting sodium atom is positively charged, for it has one less electron than protons. It becomes a sodium ion, written as Na+. The chlorine atom is negatively charged, for it has one more electron than protons. It becomes a chloride ion, written as Cl-. Note the change in name from chlorine to chloride.
As these examples suggest, an ion can be defined as an atom or a group of atoms that has acquired a positive or negative charge as a result of gaining or losing one or more electrons. By this definition molecules as well as atoms can become ions by gaining or losing electrons. Table salt consists of positively charged sodium ions and negatively charged chloride ions, each strongly attracted to the other. The attraction between oppositely charged ions forms an ionic bond.
Sodium and chlorine
can react to form the salt sodium chloride (NaCl). By losing one
electron, sodium becomes a positive ion, and by gaining one
electron, chlorine becomes a negative ion, chlorine.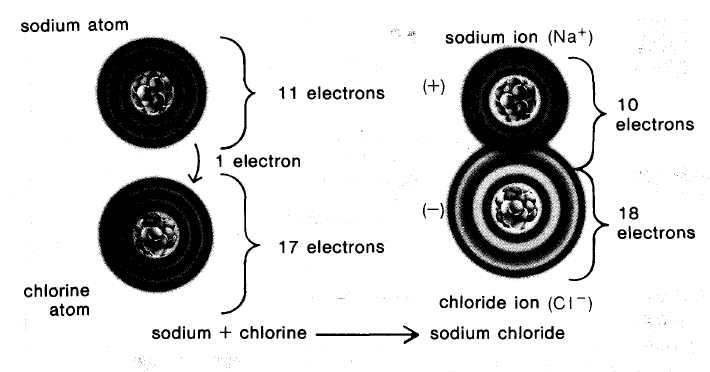
The chemical behavior of water indicates that the atoms do not share the electrons equality. The larger oxygen atom attracts the electrons more strongly than the smaller hydrogen atoms do. In the electrons of a bond are not shared equally, the bond is called a polar covalent bond. In contrast, the electrons in a molecule of hydrogen gas are shared equally, and the resulting covalent bond is said to be nonpolar. Polar molecules may form still another type of chemical bond. An attraction can occur between a slightly positive hydrogen atom in a molecule and a nearby slightly negative atom of another molecule (or of the same molecule if it is large enough).This type of attraction is called a hydrogen bond.
Exercise 7. Master the active vocabulary:
bond [bond ] - связь, соединение
electron [i´lektron ] - электрон
tissue [´tiu:] - ткань
chloride [´kl :raid] - хлорид
sodium [´s∂ di∂m] -натрий
chlorine [´kl :ri:n ] - хлор
ion [´ai∂n] -ион
proton [´pr∂ ton] - протон
Exercise 8. Answer the questions:
1) What does a chemical reaction involve?
2) In what organisms are chemical reactions always occuring?
3) What do substances form in chemical reactions?
4) What does the making of bonds involve?
5) How many types of chemical bonds can atoms form when they interact? Enumerate them.
6) What does table salt consist of?
7) What does the chemical behavior of water indicate?
8) What type of attraction is called a hydrogen bond?
Exercise 9. Put an appropriate expression in each blank.
1. When two or more atoms ............. compounds, chemical bonds are formed by ............., or transfer to outer electrons from one atom to other.
2. In chemical reactions, substances ............. and ............. new bonds and new substances.
3. As a result of chemical reactions in a cell, energy ............. used to do work, or released.
4. The resulting sodium atom ............, for it has one less electron than protons.
5. The attraction between ............. forms an ionie bond.
