
Therapeutic Micro-Nano Technology BioMEMs - Tejlal Desai & Sangeeta Bhatia
.pdf204 |
AMY POPE-HARMAN AND MAURO FERRARI |
the development of cancer [6, 75]. Even single nucleotide polymorphisms are recognizable using current biochip technology [12]. We know that the number of identifiable genetic abnormalities increases with progression to cancer from pre-cancerous risk and that early intervention through re-instatement of absent or malfunctioning genetic material is effective in averting the transition to cancer [52, 73].
Nanoparticulates can be paired with existing imaging modalities to aide in visualization of cancerous tissue and its characteristics. The redox state of specific biologic molecules in living systems [20, 22, 50] can be determined. Additionally, conjugation of folate and polyethylene glycol onto superparamagnetic particles results in increased uptake in cancer cells [95]. These may be imaged by MRI. Areas of tumor heterogeneity can be identified by contrast-enhanced MRI [19]. Gadolinium nanoparticles (folate and polyethylene glycol-coated) may be useful for both imaging and treatment through neutron capture therapy, and have been demonstrated to localize to tumors in vivo [74]. Iodinated nanoparticles have successfully localized to lymph nodes after bronchoscopic instillation, and may be imaged by CT [58]. Quantum dots, visible on stimulation with discrete light wavelengths, can be targeted to protein markers in the lung endothelium and to cancers [1]. Tissue proteomic techniques may be employed to identify the early protein markers of cancer [38].
Many types of biodegradable nanoparticles for the purpose of therapeutic agent delivery have been manufactured [79, 96]. Polymeric nanoparticles have been used to achieve gene transfection within tumor cells [77]. Nanoparticles may serve to localize drug delivery and to avoid first-pass metabolism [4]. In fact, certain nanoparticles may be able to partially evade the immediate recognition of the recipient’s immune system, allowing the potential for a longer time of efficacy and improved arrival at target tissues [68]. Nigavekar and colleagues have taken initial steps toward targeting nanodendrimers, which may serve as drug delivery devices, to tumors [70]. Polymer nanoparticles have brought about enhanced uptake of immuno-active drugs (cyclosporin) into macrophages [89]. These are just a few examples of many promising technologies in current development in the fight against the pain and suffering due to lung and other cancers.
11.3.3.2. Pulmonary Thromboembolic Disease Pulmonary thromboembolic (PTE) disease is a potential killer that is common in both the hospitalized and non-hospitalized patient. It involves formation of clot, usually in the large slow-flowing veins of the legs and pelvis, followed by dislodgement of recently-formed clot into venous flow to travel through the right heart and into the pulmonary vasculature. PTEs kill by both occluding the pulmonary vasculature, thus impairing the ability of the normally-weak right heart to provide sufficient blood return to the systemic (left) heart and by causing profound decreases in blood oxygen levels. The diagnosis of PTE is fraught with difficulty. The ”gold standard” is still considered to be pulmonary angiogram, a procedure that involves threading a catheter into the pulmonary arteries in question, which requires a skilled invasive radiologist to perform and interpret. Smaller clots are not easily detected by this or other means. With the more recently-recognized CT angiogram, accurate results depend upon correct timing and technique of intravenous dye injection and are still subject to misinterpretation by radiologists of lesser familiarity with the technique.
Nanotechnology may provide help in this realm as well. Nanoparticles conjugated to proteins that recognize mature clot (i.e. fibrin) could be constructed to be identified by common radiographic modalities, thus allowing more accurate diagnosis. Were thrombolytic
MEDICAL NANOTECHNOLOGY AND PULMONARY PATHOLOGY |
205 |
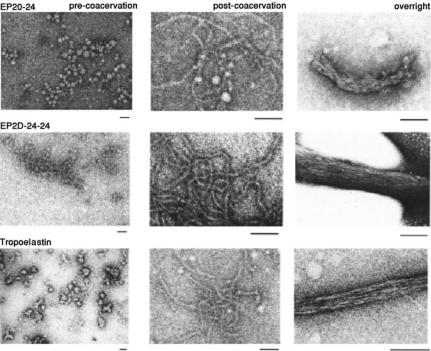
FIGURE 11.6. Comparison of structures formed from EP20-24, EP20-24-24, and tropoelastin at three stages of coacervation. Precoacervation and immediately (10 min) following coacervation (“postcoacervation”)the structures formed by the polypeptides are similar to those formed by tropoelastin. However, after overnight incubation above the coacervation temperature (“overnight”) the structures formed from EP20-24-24 closely resemble that of tropoelastin, whereas structures formed from EP20-24 are less compact and well-organized. All scale bars represent 100 nm [7, 11].
medications to be packaged within fibrin-targeting nanoliposomes, the clot-lysis medication could be released specifically at or near the site of the obstructing clot by a technique as simple as ultrasound directed at the area of clot. The nanoliposomes containing the potentially-dangerous thrombolytics are disrupted by the ultrasound at the site of the clot and re-form when they are not under the influence of ultrasound. Thrombolytics are released from the disrupted liposomes only into the area around the clot. Liposomes that target clots that are not involved in the embolic event do not release their payload. Such site specificity could allow repeated treatments and more concentrated thrombolysis at the site of the clot burden, which could provide greater success in resolution of particularly large and mature clots [33]. Kim Hamad-Schifferli’s innovation of on-off regulation of chemical events by application of localized magnetic fields to achieve temporary denaturation of proteins after administration of protein-conjugated metallic nanoparticulates may be applicable to this and other, more precise biologic localization and control applications [45]. Certain polymers change their configuration with changes in pH. Were these polymers configured to contain thrombolytics or other medication while in a higher-pH environment and release in areas of lower pH, the medication could be released at the site of clot (with its subsequent
206 |
AMY POPE-HARMAN AND MAURO FERRARI |
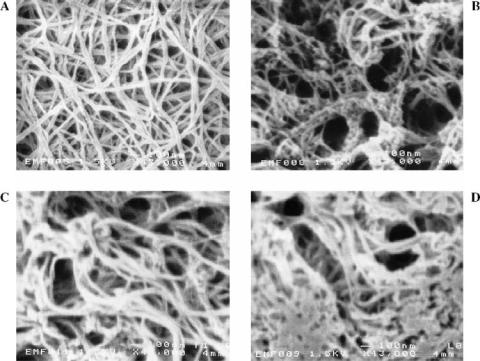
FIGURE 11.7. SEMs of collagen with chitosan of different proportions where collagen concentration is always 8.0 mg/ml: (A) pure collagen matrices; (B) collagen–chitosan composite matrices with 1:1 proportion; (C) collagen– chitosan matrices with 1:3 proportion; and (D) collagen-chitosan matrices with 1:3 proportion and with K562 cells in three-dimensional gel. (Original magnification: 343,000.) [87]
ischemia), where the pH would be expected to be relatively lower owing to the anaerobic environment. Were there difficulty in re-establishing adequate circulation or oxygenation after or during PTE, nanoparticles may be able to enhance oxygen delivery in thrombotic states [15].
11.3.3.3. Asthma Simple asthma may be diagnosed by pulmonary function testing, which demonstrates diminished exhalation (“obstruction”) during exacerbations. This abnormality usually improves to normal between episodes. Some patients, though, who have severe or chronic asthma, never return to normal breathing. Although there may be many causes of this lack of normalization between asthma exacerbations, it has been recognized over the past several years that airway remodeling, or a change in the structure of the underlying tissues that form the air passages, may be responsible. Airway remodeling is characterized by thickened smooth muscle and subepithelium, increases in collagens III and V, tenascin, laminin, and fibrin in the sub-basement membrane, as well as matrix metaloproteins and secretion by the smooth muscle of immunomodulating cytokines [53, 76]. Currently, there is no way to quantify this response aside from intra-airway biopsies, which are difficult to perform and pose some risks. Ultrastructure-Accounting Characterization
MEDICAL NANOTECHNOLOGY AND PULMONARY PATHOLOGY |
207 |
Mode Ultrasound [64] via bronchoscopy may provide information regarding both the underlying tissue structure of the airways as well as the particular composition and presence of inflammatory indicators through the use of targeted contrast agents.
11.3.3.4.Pulmonary Infections Pulmonary infections are common and account for significant morbidity and mortality [34, 72]. Even under the most ideal circumstances, we can determine culprit organism(s) less than 50% of the time [69]. This lack of information regarding responsible infectious agents not only prevents patients from receiving optimal antibiotics sooner (especially in the case of antibiotic-resistant organisms), but also contributes to the problem of antibiotic resistance by forcing empiric initiation of available antibiotics in patients who are too ill to wait for results of cultures and sensitivities. A means by which we could both quickly identify specific organisms and determine their sensitivities to antibiotics would likely result in significant improvement in pneumoniarelated morbidity and mortality [51]. The specific organisms as well as the presence of any resistance-conferring genetic material could be quickly identified by use of lab-on-a-chip microsystems [90], among other means.
Prevention of pulmonary infections, making use of the body’s inherent mechanisms of directed humorally-mediated protection, may be even more appealing than attempting to treat an already-established problem. Vaccines hold promise as well for the policing of abnormal cells that may develop into cancers. The immunogenicity of biologic polymers may be optimized either for use as vaccines [34] or, as in the case of conjugated nanoparticulates for drug delivery, for avoidance of immune response [62]. G Ferrari with the author and SC Lee, in unpublished calculations, have developed programming by which optimal sequences of proteins may be manufactured to allow immunologic response to multiple antigenic sites at once. Polymer nanoparticulates in the form of chitosan nanoparticles may provide a means of bringing about unique topical (epithelial) genetic immunization [21].
11.3.3.5.Pulmonary Fibrosis Idiopathic pulmonary fibrosis is a devastating pulmonary disorder that often results in death within a few years [29]. The only recourse that some patients have is lung transplantation, which is not an ideal solution, and one that is not open to many of this generally-older patient population. Among other therapies, interferon gamma has been utilized with some success to delay the inevitable deterioration of this disease [94]. Interferon gamma results in stimulation of the immune system and is poorly tolerated by some patients, especially the elderly, causing malaise, fevers, and occasional low blood pressure. An implantable drug delivery device for the administration of interferon is currently under development for use in anti-cancer therapy [63] and may similarly be applicable to use in idiopathic pulmonary fibrosis, allowing therapy to maintain efficacy without as many of the side effects.
11.4.CONCLUSION
In conclusion, we have briefly introduced many of the common lung diseases we face as a population. Potential barriers to the development of new medical practices were discussed. Pitfalls that may be encountered when accessing and manipulating the lungs were clarified. We have discussed nanotechnology as it is already introduced into pulmonary medical
208 |
AMY POPE-HARMAN AND MAURO FERRARI |
practice. Lastly, we have presented a few of the many potential applications—both near at hand and as projections for the future—of nanotechnology toward the most common and devastating problems of the pulmonary system.
There are many opportunities in medicine, advances yet to be made, problems to be tackled. Nanotechnology and its offshoots may provide the means to greatly improve patient care and life in general. It will require, though, continued hard work and collaboration. People with specialized technologic knowledge are not necessarily present to see the needs of the patients. Clinicians must remain alert to the problems that they see at the bedside rather than merely accepting the status quo. The clinical problems must then be brought to the engineers and the chemists and the physicists, a dialogue established so that they may begin to apply their expertise to together find acceptable solutions. We are fully encouraging of these collaborations. This interaction is most critical in bringing about further advances, though is sometimes challenging as well, requiring that differences in language and constraints of time both be overcome. Interdisciplinary training for the future professionals in these areas will be crucial in maintaining the momentum toward further advances.
REFERENCES
[1]M.E. Akerman, W.C. Chan, P. Laakkonen, S.N. Bhatia, and E. Ruoslahti. Nanocrystal targeting in vivo. Proc. Natl. Acad. Sci. U.S.A., 99(20):12617–12621, 2002.
[2]M.G. Allen. www.cmmt.gatech.edu, Center for MEMS and Microsystems Technologies, Georgia Institute of Technology, 2002.
[3]F.A. Anderson Jr., H.B. Wheeler, and R.J. Goldberg. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism: theworcester DVT study. Arch. Intern. Med., 151:933–938, 1991.
[4]P. Arbos, M.A. Campanero, M.A. Arangoa, and J.M. Irache. Nanoparticles with specific bioadhesive properties to circumvent the pre-systemic degradation of fluorinated pyrimidines. J. Control Rel., 96(1):55–65, 2004.
[5]C. Beaulac, S. Clement-Major, J. Hawari, and J. Lagace. Eradication of mucoid Pseudomonas aeruginosa with fluid liposome-encapsulated tobramycin in an animal model of chronic pulmonary infection. Antimicrob. Agents Chemother., 40:665–669, 1996.
[6]S.A. Belinsky, K.J. Nikula, W.A. Palmisano, R. Michels, G. Saccomanno, E. Gabrielson, S.B. Baylin, and J.G. Herman. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc. Natl. Acad. Sci. U.S.A., 95(20):11891–11896, 1998.
[7]C.M. Bellingham, M.A. Lillie, J.M. Gosline, G.M. Wright, B.C. Starcher, A.J. Bailey, K.A. Woodhouse, and F.W. Keeley. Recombinant human elastin polypeptides self-assemble into biomaterials with elastin-like properties, Biopolymers, 70(4):445–455, 2003.
[8]Y. Benenson, B. Gil, U. Ben-Dor, R. Adar, and E. Shapiro. An autonomous molecular computer for logical control of gene expression. Nature, 429(6990):423–429, 2004.
[9]P.J. Borm. Particle toxicology: from coal mining to nanotechnology. Inhal. Toxicol., 14(3):311–324, 2002.
[10]H. Bounameaux, L. Hicklin, and S. Desmarais. Seasonal variation in deep vein thrombosis. BMJ, 312:284– 285, 1996.
[11]G. Bressan, I. Pasquali-Ronchetti, C. Fornieri, F. Mattioli, I. Castellani, and D. Volpin. J. Ultrastruct. Res., 94:209–216, 1986.
[12]J. Burmeister, V. Bazilyanska, K. Grothe, B. Koehler, I. Dorn, B.D. Warner, and E. Diessel. Single nucleotidepolymorphism analysis by chip-based hybridization and direct current electrical detection of gold-labeled DNA. Anal. Bioanal. Chem., 379(3):391–398, 2004.
[13]T. Burnouf, Radosevich. Nanofiltration of plasma-derived biopharmaceutical products. Haemophilia, 9:24– 37, 2003.
[14]E.L. Chaikof, H. Matthew, J. Kohn, A.G. Mikos, G.D. Prestwich, and C.M. Yip. Biomaterials and scaffolds in reparative medicine. Ann. NY Acad. Sci., 961:96–105, 2002.
MEDICAL NANOTECHNOLOGY AND PULMONARY PATHOLOGY |
209 |
[15]C. Chauvierre, M.C. Marden, C. Vauthier, D. Labarre, P. Couvreur, and L. Leclerc. Heparin coated poly(alkylcyanoacrylate) nanoparticles coupled to hemoglobin: a new oxygen carrier. Biomaterials, 25(15):3081–3086, 2004.
[16]R.J. Chen H.C. Choi, S. Bangsaruntip, E. Yenilmez, X. Tang, Q. Wang, Y.L. Chang, and H. Dai. An investigation of the mechanisms of electronic sensing of protein adsorption on carbon nanotube devices. J. Am. Chem. Soc., 126(5):1563–1568, 2004.
[17]W.W. Coon. Epidemiology of venous thromboembolism. Ann. Surg., 186:149–164, 1977.
[18]C. Cordeiro, D.J. Wiseman, P. Lutwyche, M. Uh, J.C. Evans, B.B. Finlay, and M.S. Webb. Antibacterial efficacy of gentamicin encapsulated in pH-sensitive liposomes against an in vivo Salmonella enterica serovar Typhimurium intracellular infection model. Antimicrob. Agents Chemother., 44:533–539, 2000.
[19]N.G. Costouros, D. Lorang, Y. Zhang, M.S. Miller, F.E. Diehn, S.M. Hewitt, M.V. Knopp, K.C. Li, P.L. Choyke, H.R. Alexander, and S.K. Libutti. Microarray gene expression analysis of murine tumor heterogeneity defined by dynamic contrast-enhanced MRI. Mol. Imaging, 1(3):301–308, 2002.
[20]J.E. Crowther, V.K. Kutala, P. Kuppusamy, J.S. Ferguson, A.A. Beharka, J.L. Zweier, F.X. McCormack, and L.S. Schlesinger. Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J. Immunol., 172(11):6866–6874, 2004.
[21]Z. Cui and R.J. Mumper. Chitosan-based nanoparticles for topical genetic immunization. J. Control Rel., 75(3):409–419, 2001.
[22]M.C. Daniel, J. Ruiz, S. Nlate, J.C. Blais, and D. Astruc. Nanoscopic assemblies between supramolecular
redox active metallodendrons and gold nanoparticles: synthesis, characterization, and selective recognition of H2PO4-, HSO4-, and adenosine-5 -triphosphate (ATP2-) anions. J. Am. Chem. Soc., 125(9):2617–2628,
2003.
[23]J.J. Davis, K.S. Coleman, B.R. Azamian, C.B. Bagshaw, and M.L. Green. Chemical and biochemical sensing with modified single walled carbon nanotubes. Chemistry, 9(16):3732–3739, 2003.
[24]P. Decuzzi, S. Lee, M. Decuzzi, and M. Ferrari. Adhesion of microfabricated particles on vascular endothelium: a parametric analysis. Ann. Biomed. Eng., 32(6):793–802, 2004.
[25]M.B. Dolovich and M.T. Newhouse. Aerosols. Generation, methods of administration, and therapeutic applications in asthma. In E. Middleton Jr., C.E. Reed, E.F. Ellis, N.F. Adkinson Jr, J.W. Yunginger, and W.W. Busse(eds.), Allergy. Principles and practice, (4th Edn.), St Louis, Mosby Year Book, Inc., pp. 712–739, 1993.
[26]P. Demaeyer, E.M. Akodad, E. Gravet, P. Schietecat, J.P. Van Vooren, A. Drowart, J.C. Yernault, and F.J. Legros. Disposition of liposomal gentamicin following intrabronchial administration in rabbits. J. Microencapsul., 10:77–88, 1993.
[27]S.V. Dzyadevych, A.P. Soldatkin, Y.I. Korpan, V.N. Arkhypova, A.V. El’skaya, J.M. Chovelon, C. Martelet, and N. Jaffrezic-Renault. Biosensors based on enzyme field-effect transistors for determination of some substrates and inhibitors. Anal. Bioanal. Chem., 377(3):496–506, 2003.
[28]M.W. Epperly, H.L. Guo, M. Jefferson, S. Nie, J. Gretton, M. Bernarding, D. Bar-Sagi, H. Archer, and J.S. Greenberger. Cell phenotype specific kinetics of expression of intratracheally injected manganese superoxide dismutaseplasmid/ liposomes (MnSOD-PL) during lung radioprotective gene therapy. Gene Ther., 10(2):163– 171, 2003.
[29]J.M. Fellrath and R.M. du Bois. Idiopathic pulmonary fibrosis/cryptogenic fibrosing alveolitis. Clin. Exp. Med., 3(2):65–83, 2003.
[30]M. Ferrari. Therapeutic Microdevices and Methods of Making and Using Same, U.S. Patent No. 6,107,102, August 22, 2000.
[31]M. Ferrari et al. Particles for Oral Delivery of Peptides and Proteins, US Patent No. 6,355,270, March 12, 2002.
[32]Ferrari. The hallmarks of cancer nanotechnology. Nature Reviews Cancer, 3(5):161–171, 2005.
[33]M. Ferrari, and P. Goldschmidt. Personal communication, 2001.
[34]T. Fifis, A. Gamvrellis, B. Crimeen-Irwin, G.A. Pietersz, J. Li, P.L. Mottram, I.F. McKenzie, and M. Plebanski. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol., 173(5):3148–3154, 2004.
[35]R.M. Fielding, R.O. Lewis, and L. Moon-McDermott. Altered tissue distribution and elimination of amikacin encapsulated in unilamellar, low-clearance liposomes (MiKasome). Pharm. Res., 15:1775–1781, 1998.
210 |
AMY POPE-HARMAN AND MAURO FERRARI |
[36]R.M. Fielding, L. Moon-McDermott, R.O. Lewis, and M.J. Horner. Pharmacokinetics and urinary excretion of amikacin in low-clearance unilamellar liposomes after a single or repeated intravenous administration in the rhesus monkey. Antimicrob. Agents Chemother., 43:503–509, 1999.
[37]G. Hernandez, P. Rico, E. Diaz, and J. Rello. Nosocomial lung infections in adult intensive care units. Microbes. Infect., 6(11):1004–1014, 2004.
[38]D.H. Geho, N. Lahar, M. Ferrari, E.F. Petricoin, and L.A. Liotta. Opportunities for nanotechnology-based innovation in tissue proteomics. Biomed. Microdev., 6(3):231-239, 2004.
[39]B.E. Gilbert. Liposomal aerosols in the management of pulmonary infections. J. Aerosol. Med., 9(1):111–122, 1996.
[40]R.F. Gillum. Pulmonary embolism and thrombophlebitis in the United States, 1970–1985. Am. Heart J., 114:1262–1264, 1987.
[41]S. Giovagnoli, P. Blasi, C. Vescovi, G. Fardella, I. Chiappini, L. Perioli, M. Ricci, and C. Rossi. Unilamellar vesicles as potential capreomycin sulfate carriers: preparation and physicochemical characterization. AAPS Pharm. Sci. Tech., 4(4):69, 2003.
[42]S.Z. Goldhaber and H. Bounameaux. Thrombolytic therapy in pulmonary embolism. Semin. Vascul. Med., 1(2):213–220, 2001.
[43]J.D. Green. Pharmaco-toxicological expert report Pulmozyme rhDNase Genentech, Inc. Hum. Exp. Toxicol., 13(Suppl 1):S1–S42, 1994.
[44]F. Hafner. Cytosensor R Microphysiometer: technology and recent applications. Biosens. Bioelectron., 15:149–158, 2000.
[45]K. Hamad-Schifferli, J.J. Schwartz, A.T. Santos, S. Zhang, and J.M. Jacobson. Remote electronic control of DNA hybridization through inductive coupling to an attached metal nanocrystal antenna. Nature, 415(6868):152–155, 2002.
[46]S. Hashimoto, J.-F. Pittet, K. Hong, H. Folkesson, G. Bagby, L. Kobzik, C. Frevert, K. Watanabe, S. Tsurufuji, and J. Wiener-Kronish. Depletion of alveolar macrophages decreases neutrophil chemotaxis to Pseudomonas airspace infections. Am. J. Physiol. (Lung Cell Mol. Physiol.), 270(14):L819–L828, 1996.
[47]G. Hernandez, P. Rico, E. Diaz, and J. Rello. Nosocomial lung infections in adult intensive care units. Microbes Infect., 6(11): 1004–14, 2004.
[48]L. Hood, J.R. Heath, M.E. Phelps, and B. Lin. Systems biology and new technologies enable predictive and preventative medicine. Science, 306(5696):640–643, 2004.
[49]O.R. Hung, S.C. Whynot, J.R. Varvel, S.L. Shafer, and M. Mezei. Pharmacokinetics of inhaled liposomeencapsulated fentanyl. Anesthesiology, 83:277–284, 1995.
[50]G. Ilangovan, A. Bratasz, H. Li, P. Schmalbrock, J.L. Zweier, and P. Kuppusamy. In vivo measurement and imaging of tumor oxygenation using coembedded paramagnetic particulates. Magn. Reson. Med., 52(3):650– 657, 2004.
[51]M. Iregui, S. Ward, G. Sherman, V.J. Fraser, and M.H. Kollef. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest, 122:262–268, 2001.
[52]H. Ishii, K.R. Dumon, A. Vecchione, L.Y. Fong, R. Baffa, K. Huebner, and C.M. Croce. Potential cancer therapy with the fragile histidine triad gene: review of the preclinical studies. JAMA, 286(19):2441–2449, 2001.
[53]P.K. Jeffery. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med., 164(10Pt 2):S28–S38, 2001.
[54] E. Jin, M. Fujiwara, M. Nagashima, H. Shimizu, M. Ghazizadeh, X. Pan, S. Arai, Y. Ohaki, M. Gomibuchi, T. Takemura. and O. Kawanami. Aerogenous spread of primary lung adenocarcinoma induces ultrastructural remodeling of the alveolar capillary endothelium. Hum. Pathol., 32(10):1050–1058, 2001.
[55]M. Joshi and A. Misra. Dry powder inhalation of liposomal ketotifen fumarate: formulation and characterization. Int. J. Pharm., 223(1-2):15–27, 2001.
[56]E. Katz and I. Willner. Biomolecule-functionalized carbon nanotubes: applications in nanobioelectronics. Chemphyschemistry, 5(8):1084–1104, 2004.
[57]G. Kersten and H. Hirschberg. Antigen delivery systems. Expert Rev. Vaccines, 3(4):453–462, 2004.
[58]L.H. Ketai, B.A. Muggenberg, G.L. McIntire, E.R. Bacon, R. Rosenberg, P.E. Losco, J.L. Toner, K.J. Nikula, and P. Haley. CT imaging of intrathoracic lymph nodes in dogs with bronchoscopically administered iodinated nanoparticles. Acad. Radiol., 6(1):49–54, 1999.
MEDICAL NANOTECHNOLOGY AND PULMONARY PATHOLOGY |
211 |
[59]A. Kierkegaard. Incidence and diagnosis of deep vein thrombosis associated with pregnancy. Acta. Obstet. Gynecol. Scand., 62:239–243, 1983.
[60]C. Khanna, P.M. Anderson, D.E. Hasz, E. Katsanis, M. Neville, and J.S. Klausner. Interleukin-2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer, 79(7):1409– 1421, 1997.
[61]W. Kruse, W. Eggert-Kruse, J. Rampmaier, B. Runnebaum, and E. Weber. Dosage frequency and drugcompliance behaviour–a comparative study on compliance with a medication to be taken twice or four times daily. Eur. J. Clin. Pharmacol., 41(6):589–592, 1991.
[62]S.C. Lee, R. Parthasarathy, K. Botwin, D. Kunneman, E. Rowold, G. Lange, J. Klover, A. Abegg, J. Zobel,
T.Beck, T. Miller, W. Hood, J. Monahan, J.P. McKearn, R. Jansson, and C.F. Voliva. Biochemical and immunological properties of cytokines conjugated to dendritic polymers. Biomed. Microdev., 6(3):191–202, 2004.
[63]G. Lesinski, S. Sharma, K. Varker, P. Sinha, M. Ferrari, and W. Carson. Release of Biologically Functional InterferonAlpha from a Nanochannel Delivery System. (in review), 2005.
[64]J. Liu and M. Ferrari. Mechanical spectral signatures of malignant disease? A small-sample, comparative study of continuum vs. nano-biomechanical data analyses. Dis. Markers, 18(4):175–183, 2002.
[65]J. Lu and Z. Rosenzweig. Nanoscale fluorescent sensors for intracellular analysis. Fresenius J. Anal. Chem., 366(6-7):569–575, 2000.
[66]J.-F. Marier, J. Lavigne, and M.P. Ducharme. Pharmacokinetics and efficacies of liposomal and conventional formulations of tobramycin after intratracheal administration in rats with pulmonary. Burkholderia cepacia infection. Antimicrob. Agents Chemother., 46(12):3776–3781, 2002.
[67]T. Minko, A. Stefanov, and V. Pozharov. Lung edema clearance: 20years of progress selected contribution: lung hypoxia: antioxidant and antiapoptotic effects of liposomal a´-tocopherol. J. Appl. Physiol., 93:1550– 1560, 2002.
[68]S.M. Moghimi, A.C. Hunter, and J.C. Murray. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev., 53(2):283–318, 2001.
[69]M.S. Niederman, L.A. Mandell, A. Anzueto, J.B. Bass, W.A. Broughton, G.D. Campbell, N. Dean, T. File, M.J. Fine, P.A. Gross, F. Martinez, T.J. Marrie, J.F. Plouffe, J. Ramirez, G.A. Sarosi, A. Torres, R. Wilson, and V.L. Yu. American thoracic society guidelines for the management of adults with community-acquired pneumonia: Diagnosis, assessment of severity, antimicrobial therapy, and prevention. Am. J. Respir. Crit. Care Med., 163(7):1730–1754, 2004.
[70]S.S. Nigavekar, L.Y. Sung, M. Llanes, El-Jawahri, T.S. Lawrence, C.W. Becker, L. Balogh, and M.K. Khan. 3H dendrimer nanoparticle organ/tumor distribution. Pharm. Res., 21(3):476–483, 2004.
[71]A. Omri, C. Beaulac, M. Bouhajib, S. Montplaisir, M. Sharkawi, and J. Lagace. Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob. Agents Chemother., 38:1090–1095, 1994.
[72]J.J. Oosterheert, M.J. Bonten, E. Hak, M.M. Schneider, and I.M. Hoepelman. How good is the evidence for the recommended empirical antimicrobial treatment of patients hospitalized because of community-acquired pneumonia? A systematic review. J. Antimicrob. Chemother., 52(4):555–563, 2003.
[73]M. Ottey, S.Y. Han, T. Druck, B.L. Barnoski, K.A. McCorkell, C.M. Croce, C. Raventos-Suarez, C.R. Fairchild, Y.Wang, and K. Huebner. Fhit-deficient normal and cancer cells are mitomycin C and UVC resistant. Br. J. Cancer, 91(9):1669–1677, 2004.
[74]M.O. Oyewumi, R.A. Yokel, M. Jay, T. Coakley, and R.J. Mumper. Comparison of cell uptake, biodistribution and tumor retention of folate-coated and PEG-coated gadolinium nanoparticles in tumor-bearing mice.
J.Control Rel., 95(3):613–626, 2004.
[75]W.A. Palmisano, K.K. Divine, G. Saccomanno, F.D. Gilliland, S.B. Baylin, J.G. Herman, and S.A. Belinsky. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res., 60(21):5954– 5958, 2000.
[76]R.A. Panettieri Jr. Airway smooth muscle: immunomodulatory cells that modulate airway remodeling?
Respir. Physiol. Neurobiol., 137(2–3):277–293, 2003.
[77]S. Prabha and V. Labhasetwar. Critical determinants in PLGA/PLA nanoparticle-mediated gene expression. Pharm. Res., 21(2):354–364, 2004.
[78]L.A.G. Ries, M.P. Eisner, C.L. Kosary, B.F. Hankey, B.A. Miller, L. Clegg, A. Mariotto, E.J. Feuer, and B.K. Edwards. SEER Cancer Statistics Review, 1975–2001, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975 2001/, 2004.
212 |
AMY POPE-HARMAN AND MAURO FERRARI |
[79]M. Roser, D. Fischer, and T. Kissel. Surface-modified biodegradable albumin nanoand microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur. J. Pharm. Biopharm., 46(3):255–263, 1998.
[80]E.J. Ruijgrok and E.W.M. VultoAGandVan Etten. Efficacy of aerosolized amphotericin B desoxycholate and liposomal amphotericin B in the treatment of invasive pulmonary aspergillosis in severely immunocompromised rats. J. Antimicrob. Chemother., 48:89–95, 2001.
[81]D.V. Schidlow. Maintaining the horizontal line: early intervention and prevention of CF lung disease. J. Cyst. Fibros., 3(2):63–66, 2004.
[82]M.J. Schoning and A. Poghossian. Recent advances in biologically sensitive field-effect transistors (BioFETs). Analyst, 127(9):1137–1151, 2002.
[83]M.D. Silverstein, J.A. Heit, and D.N. Mohr. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch. Intern. Med., 158:585–593, 1998.
[84]P.M. Sinha, G.J. Valco, S. Sharma, X. Liu, and M. Ferrari. Nanoengineered device for drug delivery application. Nanotechnology, 15:S585–S589, 2004.
[85]A. Stikeman. The Programmable Pill, MIT Technology Review, May, 78–82.
[86]Z.E. Suntres and P.N. Shek. Incorporation of alpha-tocopherol in liposomes promotes the retention of liposomeencapsulated glutathione in the rat lung. J. Pharm. Pharmacol., 46:23–28, 1994.
[87]W. Tan, R. Krishnaraj, and T.A. Desai. Evaluation of nanostructured composite collagen–chitosan matrices for tissue engineering. Tissue Eng., 7(2):203–210, 2001.
[88]G. Viegi, A. Scognamiglio, S. Baldacci, F. Pistelli, and L. Carrozzi. Epidemiology of chronic obstructive pulmonary disease (COPD). Respiration, 68(1):4–19, 2001.
[89]J. Wang and Q. Zhang. Uptake of cyclosporineAloaded colloidal drug carriers by mouse peritoneal macrophages in vitro. Acta Pharmacol. Sin., 22(1):57–61, 2001.
[90]Z. Wang J. El-Ali, M. Engelund, T. Gotsaed, I.R. Perch-Nielsen, K.B. Mogensen, D. Snakenborg, J.P. Kutter, and A. Wolff. Measurements of scattered light on a microchip flow cytometer with integrated polymer based optical elements. Lab. Chip., 4(4):372–377, 2004.
[91]K.B. Weiss and D.K.S.O. Wagener. Asthma surveillance in the United States: a review of current trends and knowledge gaps. Chest, 98(5 Suppl):179S–184S, 1990.
[92]K.B. Weiss, P.J. Gergen, and E.F.S.O. Crain. Inner-city asthma: the epidemiology of an emerging US public health concern. Chest, 101(6 Suppl):362S–367S, 1992.
[93]T. Yokoyama, K. Murai, T. Murozuka, A. Wakisaka, M. Tanifuji, N. Fujii, and T. Tomono. Removal of small non-enveloped viruses by nanofiltration. Vox Sang., 86(4):225–229, 2004.
[94]R. Ziesche, E. Hofbauer, K. Wittmann, V. Petkov, and L.H. Block. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N. Engl. J. Med., 341(17):1264–1269, 1999.
[95]Y. Zhang, N. Kohler, and M. Zhang. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials, 23(7):1553–1561, 2002.
[96]F. Zhao, Y. Yin, W.W. Lu, J.C. Leong, W. Zhang, J. Zhang, M. Zhang, and K. Yao. Preparation and histological evaluation of biomimetic three-dimensional hydroxyapatite/chitosan-gelatin network composite scaffolds. Biomaterials, 23(15):3227–3234, 2002.
12
Nanodesigned Pore-Containing Systems for Biosensing and Controlled Drug Release
Fr´ed´erique Cunin,a Yang Yang Li,b and Michael J. Sailorb
aDr. F. Cunin, UMR CNRS/ENSCM 5618, 8 rue de l’ecole´ normale, 34296 Montpellier cedex 5, France
bProf. M. J. Sailor, Yang Yang Li, Department of Chemistry and Biochemistry, The University of California, San Diego, 9500 Gillman Drive, La Jolla, CA 92039-0358, USA
For medical treatment of disease, optimal therapeutic efficiency of a drug is governed by both the therapeutic activity of the drug itself and the way in which it is delivered to the patient. Once administered, it is as crucial to control the rate at which a drug is released in the body as it is to control its transport to the desired location. Numerous systems have been introduced over the past three decades to get drugs into the body [1] and there are many more innovative concepts currently in development. The role that nanotechnology plays in this effort is increasing dramatically.
Whereas synthetic chemistry involves the manipulation of matter at the molecular level, nanotechnology can be thought of as a discipline in which the tools of the chemist are applied to problems whose size and level of complexity lie above the molecular level. Biology operates in this domain; a living cell is a complex assembly of interconnected molecular machines and hierarchical structures. Nanotechnology operates in the same size regime, although its tools and its applications are not restricted to biology. In particular, nanostructured porous materials offer a degree of control in both the rate and the location of drug delivery that is just beginning to be recognized. This article will survey the widely accepted methods for controlled drug delivery and then focus on nanostructured materials-in particular silicon-based photonic and templated materials as examples.
