
Environmental Biotechnology - Jordening and Winter
.pdf
7.3 Degradative Capacities of Fungi 221
covalent bonds (e.g., aryl-ether, aryl-aryl, aliphatic carbon-aryl bonds; Fengel and Wegener 1989). The polymer arises from the enzyme-initiated polymerization of phenolic precursors (coniferyl, sinapyl, p-cumaryl alcohol) via the radical coupling of their corresponding phenoxy radicals. Because of the types of bonds and their heterogeneity, lignin cannot be degraded by hydrolytic mechanisms as most other natural polymers can.
During the course of evolution, only certain basidiomycetous fungi have developed an efficient enzyme system to mineralize lignin substantially, so these microorganisms play an important role in maintaining the global carbon cycle (Griffin 1994). Because of their ability to remove lignin selectively while leaving behind white cellulose fibers, these fungi are also called white-rot fungi. Typical representatives are the wood degraders Trametes versicolor, Phanerochaete chrysosporium, Pleurotus ostreatus, and Nematoloma frowardii, as well as the litter-decomposers Agaricus bisporus, Agrocybe praecox, and Stropharia coronilla. Lignin degradation does not provide a primary source of carbon and energy for fungal growth and is therefore a cometabolic process in principle (mostly sugars released by hydrolases from hemicelluloses are used as growth substrates).
Lignin degradation is thought to be brought about by the synergistic action of several oxidoreductases (lignininolytic enzymes). Two types of enzymes, namely peroxidases (manganese peroxidase, MnP, EC 1.11.1.13; lignin peroxidase, LiP, EC 1.11.1.14) and laccase (Lacc, EC 1.10.3.2), are the key biocatalysts and are responsible for the unspecific attack on lignin (Hatakka 2001). Both peroxidases are ferric- iron–containing heme proteins requiring peroxides (e.g., H2O2) for function, and laccase belongs to the copper-containing blue oxidases that use molecular oxygen (O2). The enzymes have the common ability to catalyze one-electron oxidations, resulting in the formation of free-radical species inside the lignin polymer (Fernando and Aust 1990). Afterwards, the radicals undergo spontaneous reactions leading to the incorporation of oxygen (O2), bond cleavages, and finally, to the breakdown of the lignin molecule (Kirk and Farrell 1987).
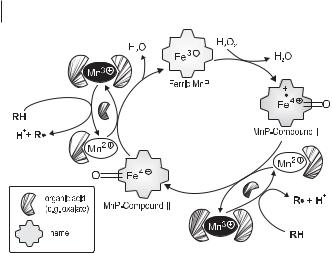
LiP and laccase react directly with aromatic lignin structures, whereas MnP works via chelated Mn3+ ions acting as low-molecular-weight redox mediators. Thus, the function of MnP is the generation of Mn3+ from Mn2+, which is the actual substrate of the enzyme. Similar to the catalytic cycle of other peroxidases, including LiP, that of MnP involves the formation of peroxidase compounds I and II. The latter are highly reactive and abstract one electron from Mn2+ to form Mn3+ (Fig. 7.11). MnP catalysis has an absolute requirement for Mn-chelating organic acids (i.e., oxalate, malate, or malonate), which both increase the affinity of Mn2+ to the enzyme and stabilize the reactive Mn3+ at high redox potentials (Wariishi et al. 1992). In contrast to the relatively large enzyme molecules, Mn3+ chelates are small enough to diffuse into the compact lignocellulosic complex, where they preferably react with phenolic lignin structures. Therefore, it is supposed that – in most white-rot fungi – the primary attack on lignin is brought about by the MnP system (Wariishi et al. 1992, Hofrichter 2002).
As the result of MnP-catalyzed primary attack, water-soluble lignin fragments are formed, which are accessible for the further conversion by ligninolytic enzymes.
222 7 Aerobic Degradation of Recalcitrant Organic Compounds by Microorganisms
Fig. 7.11 Catalytic cycle of manganese peroxidase (according to Wariishi et al. 1992; Hofrichter 2002).
Laccase oxidizes phenolic structures, whereas LiP preferentially cleaves recalcitrant nonphenolic lignin moieties (e.g., â-O-4 ethers). MnP is also involved in further degradation of the lignin fragments. Thus, there are indications that the MnP system even mineralizes aromatic lignin structures directly. It is the only enzyme – as far as known – that converts aromatic compounds, including lignin, partly to carbon dioxide (CO2). This means that lignin can be mineralized – at least in part – outside the fungal hyphae (Hofrichter 2002). The reactions underlying the MnP-catalyzed mineralization are not yet completely understood, but it is certain that decarboxylation reactions involving Mn3+ are the final step in this process. Furthermore, certain radicals (carbon-centered radicals, peroxyl radicals, superoxide) that derive from the autocatalytic decomposition of organic acids in the presence of Mn3+ may also be involved in the mineralization process.
The oxidative strength of the MnP system can be enhanced in the presence of cooxidants such as unsaturated fatty acids or thiols. These are first oxidized by Mn3+ and form – in the presence of O2 – highly reactive radical species (peroxyl and alkoxy radicals of fatty acids, thioxyl radicals), which enhance lignin mineralization and make possible the cleavage of structures that are normally not attacked by the MnP system (e.g., nonphenolic aromatic aryl ethers; Hofrichter 2002).
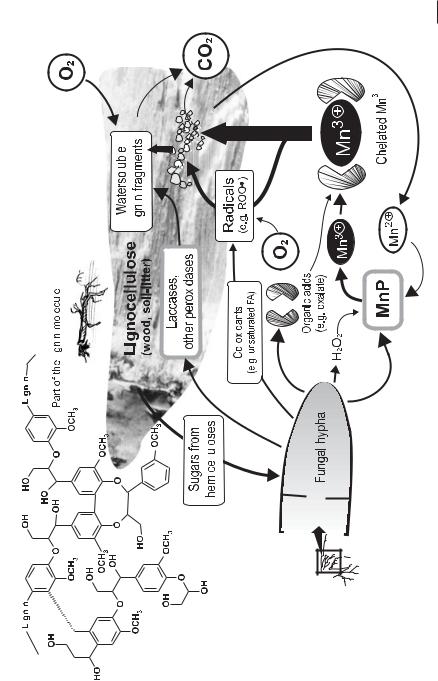
According to a concept of Kirk and Frarrell (1987), the process of MnP-catalyzed degradation of lignin and other substances has been described as ‘enzymatic combustion’, in the course of which MnP acts as a ‘radical pump’ (Fig. 7.12, Hofrichter 2002).
Finally, we should mention that recent studies have indicated that alternative ligninolytic systems may exist that use chloroperoxidases (CPOs) as key enzymes. Examples are the ascomycete Leptoxyphium (Caldariomyces) fumago and the basidiomycete Agrocybe aegerita. Both fungi colonize plant debris and produce heme-thiolate CPOs that oxidize various aromatic substrates, including aryl alcohols, nonphenolic
7.3 Degradative Capacities of Fungi 223
‘Enzymatic combustion’ of lignin by manganese-peroxidase–producing basidiomycetes. The degradation of lignin is a generally aerobic, cometabolic process. Sugars and acetate derived from hemicelluloses and glucose from cellulose serve as the actual growth substrates.

224 7 Aerobic Degradation of Recalcitrant Organic Compounds by Microorganisms
lignin model compounds, and synthetic lignin preparations (Ortiz-Bermúdez et al. 2003; Ullrich et al. 2004).
7.3.2.2 Degradation of Organopollutants
The unique ligninolytic enzyme system of basidiomycetous fungi, being based on a highly reactive free-radical depolymerization mechanism, should be ideal for the biodegradation of organopollutants in the environment (Fernando and Aust 1990). An excellent review of the feasibility of bioremediation with white-rot fungi was published by Pointing in 2001.
Compared with other potential bioremediation systems, the extracellular, nonspecific, nonstereoselective lignin-degrading system of basidiomycetous fungi has the advantage of being applicable to a variety of recalcitrant and toxic chemicals. Examples of such chemicals and selected fungi responsible for their biodegradation are given in Table 7.4. These substances include such hazardous xenobiotic compounds as polychlorinated dibenzodioxines and dibenzofurans, other chlorinated aromatics, nitroaromatic compounds (explosives), and carcinogenic organopollutants belonging to the polycyclic aromatic hydrocarbons (Hofrichter and Fritsche 2000, Manji and Ishihara 2003). MnP, LiP, and laccase have all been shown to be involved in the oxidation of organopollutants (Bollag 1988, Barr and Aust 1994). As a result of the attack on organopollutants by ligninolytic enzymes, various metabolites are formed that either can be further degraded intracellularly, coupled to the humus, or mineralized by the MnP system. The latter has been shown to mineralize several organopollutants partly in cell-free systems, indicating that an extracellular ‘enzymatic combustion’ of hazardous chemicals is possible in principle (Hofrichter 2002).
Over the last decade, efforts have been made to use ligninolytic basidiomycetes in bioremediation technologies. Laboratory experiments have shown that degradation of certain organopollutants (e.g., PAHs, PCP, TNT) is stimulated by wood-inhabit- ing white-rot fungi in contaminated soils (Phanerochaete chrysosporium, Lentinus
Table 7.4 Selected organopollutants that are transformed or mineralized by ligninolytic basidiomycetes and/or their ligninolytic enzymes (according to Barr and Aust 1994, Fritsche and Hofrichter 2000; Baciocchi et al. 2000, Haas et al. 2003).
Fungus |
Organopollutants |
|
|
Bjerkandera adusta |
Benzo(a)pyrene, other PAHs, TNT, dyes |
Nematoloma frowardii |
Benzo(a)pyrene, other PAHs, TNT, DCP, PCP, AsO |
Phanerochaete chrysosporium |
Benzo(a)pyrene, other PAHs, BTX, DNT, TNT, DDT, DCP, |
|
PCP, PCBs, DCA, dyes, polystyrenes, KCN, aromatic sulfides |
Phanerochaete sordida |
PAHs, polychlorinated DBDs and DBFs |
Phlebia radiata |
TNT, dyes |
Pleurotus ostreatus |
Benzo(a)pyrene, other PAHs, dibenzothiophene, TNT |
Stropharia rugosoannulata |
DCP, PCP, TNT |
Trametes versicolor |
Benzo(a)pyrene, other PAHs, DCA, DCP, PCP, dyes |
|
|

7.4 Conclusions 225
edodes, Kuehneromyces mutabilis; Rajarathnam et al. 1998). However, a disadvantage of these fungi is their small competitive potential in soil. Therefore, recent research has focused on litter-decomposing basidiomycetes, which naturally colonize the upper-most portion of the soil and the humus layers of forests and grasslands. Stropharia rugosoannulata, S. coronilla, Agrocybe praecox, and Collybia dryophila are examples of such fungi. Stropharia rugosoannulata has already been successfully tested for decontamination of soils containing TNT as well as for PAH removal (Fritsche et al. 2000, Steffen et al. 2003).
7.4 Conclusions
It has been said that without microorganisms and their degradative capabilities animal life, including human life, on the earth would cease to exist within about five years. Whether or not this is an exaggeration as to the time scale, it is true in principle that we absolutely depend on microbial activities for the renewal of our environment and maintenance of the global carbon cycle (Ratledge 1991). Among the substances that can be degraded or transformed by microorganisms are a huge number of synthetic compounds (xenobiotics) and other chemicals having environmental relevance (e.g., mineral oil components). However, it has to be considered that this statement concerns potential degradabilities which – in most instances – were estimated in the laboratory by using pure cultures and ideal growth conditions. Under natural conditions in soil, the actual degradability of organopollutants is lower, due to a whole range of factors: competition with other microorganisms, insufficient supply with essential substrates (C, N, P, S sources), unfavorable external conditions (O2, H2O, pH, temperature), and low bioavailability of the pollutant that is to be degraded. Thus, environmental biotechnology has the important assignment of tackling and solving these problems so as to permit the use of microorganism in bioremediation technologies. For this purpose, it is necessary to support the activities of the indigenous microorganisms in polluted soils and to enhance their degradative potential by bioaugmentation. The former measure particularly applies to bacteria, whereas bioaugmentation mainly concerns basidiomycetous fungi. In this context, we should point out that neither bacteria nor fungi are ‘better’ degraders. In nature, both groups of microorganisms work together of course, and complement one another in their degradative capabilities.

2267 Aerobic Degradation of Recalcitrant Organic Compounds by Microorganisms
References
Abramowicz, D. A., Aerobic and anaerobic biodegradation of PCBs: a review, Crit. Rev. Biotech. 1990, 10, 241–251.
Alexander, M., Biodegradation and Bioremediation, San Diego CA 1994: Academic Press.
Apajalahti, J. H. A., Salkinoja-Salonen, M. S., Complete dechlorination of terachlorohydroquinone by cell extracts of pentachlo- rophenol-induced Rhodococcus chlorophenolicus, J. Bacteriol. 1987, 169, 5125–5130.
Baciocchi, E., Gerini, F., Harvey, P. J., Lanzalunga, O., Mancinelli, S., Oxidation of aromatic sulfides by lignin peroxidase from
Phanerochaete chrysosporium. Eur. J. Biochem. 2000, 267, 2705–2710.
Barr, D. P., Aust, S. D., Mechanisms of white rot fungi to degrade pollutants, Environ. Sci. Technol. 1994, 2, 78A–87A.
Bartha, R., Biotechnology of petroleum pollutant degradation, Microbiol. Ecol. 1986, 12, 155–172.
Bollag, J.-M., Shuttleworth, K. L., Anderson, D.H., Laccase-mediated detoxification of phenolic compounds, Appl. Environ. Microbiol. 1988, 54, 3086–3091.
Britton, L. N., Microbial degradation of aliphatic hydrocarbons, in: Microbial Degradation of Organic Compounds (Gibson, D. T., ed.), pp. 89–129. New York 1984: Marcel Dekker.
Dix, N. J., Webster, J., Fungal Ecology. London 1995: Chapman & Hall.
Dutta, D., Ghosh, D. K., Mishra, A. K., Samanta, T.B., Induction of benzo(a)pyrene hydroxylase in Aspergillus ochraceus TS: evidences of multiple forms of cytochrome P- 450, Biochem. Biophys. Res. Commun. 1983,
115, 692–699.
Fengel, D., Wegener, G., Wood: Chemistry, Ultrastructure, Reactions, Berlin 1989: Walter de Gruyter.
Fernando, T. Aust, S. D., Biodegradation of toxic chemicals by white rot fungi, in: Biological Degradation and Bioremediation of Toxic Chemicals (Chaudhry, G. R., Ed.), pp. 386–402. London 1990: Chapman & Hall.
Fritsche, W., Hofrichter, M., Aerobic degradation by microorganisms, in: Biotechnology. Vol. 11b, Environmental Processes, (- Rehm, H.-J., Reed, G., Eds.), Wiley-VCH, Weinheim 2000, pp. 145–167.
Fritsche, W., Scheibner, K., Herre, A., Hofrichter, M., Fungal degradation of explosives: TNT and related nitroaromatic compounds, in: Biodegradation of Nitroorganic Compounds and Explosives. (Spain, J., Hughes, J. B., Knackmuss, H.-J., Eds.), Boca Raton FL 2000: Lewis Publishers, pp. 213–237.
Griffin, D. H., Fungal Physiology. New York 1994: Wiley-Liss.
Haas, R., Tsivunchyk, O., Steinbach, K., v. Löw, E., Scheibner, K., Hofrichter, M., Conversion of adamsite (phenarsazine chloride) by fungal manganese peroxidase. Appl. Microbiol. Biotechnol., online first: DOI: 10.1007/s00253-003-1453-x (2003).
Hatakka, A., Biodegradation of lignin, in Biopolymers. Vol. 1: Lignin, Humic Substances and Coal. (Hofrichter, M., Steinbüchel, A., Eds), Weinheim 2001: Wiley-VCH, pp. 129–180.
Henry, S. M., Grbic-Galic, D., Biodegradation of trichloroethylene in methanotrophic systems and implications for process applications, in: Biological Degradation and Bioremediation of Toxic Chemicals (Chaudhry, G. R., ed.), pp. 314–344. London 1994: Chapman & Hall.
Hofrichter, M., Bublitz, F., Fritsche, W., Unspecific degradation of halogenated phenols by the soil fungus Penicillium frequentans Bi 7/2, J. Basic Microbiol. 1994, 34, 163–172.
Hofrichter, M., Scheibner, K., Bublitz, F., Schneegaß, I., Ziegenhagen, D., Martens, R., Fritsche, W., Depolymerization of straw lignin by manganese peroxidase is accompanied by release of carbon dioxide. Holzforschung 1999, 53, 161–166.
Hofrichter, M., Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microbiol. Technol. 2002, 30, 454–466.
Hommel, R. K., Formation and physiological role of biosurfactants produced by hydrocar- bon-utilizing microorganisms, Biodegradation 1990, 1, 107–109.
Houghton, J. E., Shanley, M. S., Catabolic potential of pseudomonads: a regulatory perspective, in: Biological Degradation and Bioremediation of Toxic Chemicals (Chaudhry, G. R., ed.), pp. 11–32. London 1994: Chapman & Hall.

Kästner, M., Humification process or formation of refractory soil organic matter, in: Biotechnology Vol. 11b, 2nd edit., (Rehm, H.-J, Reed, G., eds), pp. 89–125. Weinheim 2000: Wiley-VCH.
Kästner, M., Degradation of aromatic and polyaromatic compounds, in: Biotechnology Vol. 11b, 2nd edit., (Rehm, H.-J, Reed, G., Eds), pp. 211–239. Weinheim 2000: WileyVCH.
Kirk, T. K., Farrell, R. L., Enzymatic ‘combustion’: the microbial degradation of lignin, Ann. Rev. Microbiol. 1987, 41, 465–505.
Knackmuss, H. J., Abbau von Naturund Fremdstoffen, in: Umweltbiotechnologie
(Ottow, C. G., Billingmaier, W, Eds.),
pp. 39–80. Stuttgart 1997: Gustav Fischer. Manji, S., Ishihara, A., Screening of tetra-
chlorodibenzo-p-dioxin–degrading fungi capable of producing extracellular peroxidases under various conditions. Appl. Microbiol. Biotechnol. 2003, 63, 438–444.
McAllister, K. A., Lee, H., Trevors, J. T., Microbial degradation of pentachlorophenol.
Biodegradation 1996, 7, 1–40.
Morgan, P., Watkinson, R. J., Biodegradation of components of petroleum, in: Biochemistry of Microbial Degradation (Ratledge, C., Ed.), pp. 1–31, Dordrecht 1994: Kluwer.
Mörtberg, M., Neujahr, H. Y., Uptake of phenol in Trichosporon cutaneum, J. Bacteriol. 1985, 161, 615–619.
Ortiz-Bermúdez, P., Srebotnik, E., Hammel, K. E., Chlorination and cleavage of lignin structures by fungal chloroperoxidase. Appl. Environ. Microbiol. 2003, 69, 5015–5018.
Pointing, S., Feasibility of bioremediation by white-rot fungi. Appl. Microbiol. Biotechnol.
2001, 57, 20–33.
Rajarathnam, S., Shashirekha, N. U., Bano, Z., Biodegradative and biosynthetic capacities of mushrooms: present and future strategies, Crit. Rev. Biotechnol. 1998, 18, 91–236.
References 227
Ramos, J. L., Marques, S., Timmis, K. N., Transcriptional control of the Pseudomonas TOL plasmid catabolic operons is achieved through an interplay of host factors and plas- mid-encoded regulators, Annu. Rev. Microbiol. 1997, 51, 341–73.
Ratledge, C., Editorial, in: Physiology of Biodegradative Microorganisms (Ratledge C., Ed.), pp. vii–viii. Dordrecht 1991: Kluwer.
Rehm, H. J., Reiff, I., Regulation of microbial alkane oxidation with respect to the formation of products, Acta Biotechnol. 1982, 3, 279–288 (in German).
Reinecke, W., Degradation of chlorinated aromatic compounds by bacteria: strains development, in: Biological Degradation and Bioremediation of Toxic Chemicals (Chaudhry, G.
R., Ed.), pp. 416–454. London 1994: Chapman & Hall.
Steffen, K., Hatakka, Hofrichter, M., Degradation of benzo(a)pyrene by the litter-decom- posing basidiomycete Stropharia coronilla: role of manganese peroxidase. Appl. Environ. Microbiol. 2003, 69, 3957–3964
Ullrich, R., Nüske, J., Scheibner, K., Spantzel, J., Hofrichter, M., A novel peroxidase from the agaric basidiomycete Agrocybe aegerita oxidizing aryl alcohols and aldehydes and showing chloroperoxidase activity, 2004, 70, 4575–4581.
Wariishi, H., Valli, K., Gold, M. H., Manganese(II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium: kinetic mechanism and role of chelators, J. Biol. Chem. 1992, 267, 23688–23695.
Watkinson, R. J., Morgan, P., Physiology of aliphatic hydrocarbon-degrading microorganisms, Biodegradation 1990, 1, 79–92.
Wright, J. D., Fungal degradation of benzoic acids and related compounds. World J. Microbiol. Biotechnol. 1993, 9, 9–16.

229
8
Principles of Anaerobic Degradation of Organic Compounds
Bernhard Schink
8.1
General Aspects of Anaerobic Degradation Processes
The vast majority of organic compounds produced in nature or through human manufacture is degraded aerobically, with molecular oxygen as terminal electron acceptor. As long as oxygen is available, it is the preferred electron acceptor for microbial degradation processes in nature.
Anaerobic degradation processes have always been considered inferior to aerobic degradation in their kinetics and capacities. They are thought to be slow and inefficient, especially with certain comparably stable types of substrates. Nonetheless, in certain anoxic environments, such as the cow’s rumen, the turnover of, e.g., cellulose is much faster than in the presence of oxygen, with average half-life times in the range of one day. Fermentative degradation of fibers in the rumen reaches its limit with plant tissues rich in lignin which largely withstands degradation in the absence of oxygen.
Also in waste treatment, especially with high loads of easy-to-degrade organic material, anaerobic processes have proved to be efficient and far less expensive than aerobic treatment: they require only small amounts of energy input, in contrast to treatment in aeration basins, and can produce a mixture of methane and CO2 (‘biogas’), which can be used efficiently for energy generation. This holds true for most waste materials that are easily accessible to degradation without the participation of oxygen, such as polysaccharides, proteins, fats, nucleic acids, etc. These polymers are hydrolyzed through specific extracellular enzymes, and the oligoand monomers can be degraded inside the cell through enzyme reactions similar to those known in aerobic metabolism. The specific activities of such enzymes in anaerobic cultures are in the same range (0.1–1 µmol substrate per min and mg cell protein) as those of aerobic bacteria, and thus the transformation rates per unit biomass should be equivalent.
Nonetheless, anaerobic bacteria obtain far less energy from substrate turnover than their aerobic counterparts. Whereas aerobic oxidation of hexose to six CO2 yields 2870 kJ per mol, dismutation of hexose to three CH4 and three CO2 yields on-
Environmental Biotechnology. Concepts and Applications. Edited by H.-J. Jördening and J. Winter Copyright © 2005 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
ISBN: 3-527-30585-8

230 8 Principles of Anaerobic Degradation of Organic Compounds
ly 390 kJ per mol, about 15% of the aerobic process, and this small amount of energy has to be shared by at least three different metabolic groups of bacteria (see Schink, 1997). As a consequence, they can produce far less biomass per substrate molecule than aerobes can. Their growth yields are low, and most often growth is slower than that of aerobes. Maintaining the biomass inside specifically designed reactors (fixed bed, fluidized bed reactors, Upflow Anaerobic Sludge Blanket reactors) helps to overcome the problem of low and slow biomass production in anaerobic degradation and largely uncouples substrate turnover from biomass growth (for an overview, see, e.g., Schink, 1988). These systems allow anaerobic wastewater treatment to be nearly as efficient as and less expensive than the aerobic process, with methane as a useful product; but the microbial communities in these advanced anaerobic reactors still are comparably sluggish in reacting to changes in substrate composition or in their reestablishment after accidental population losses due to toxic ingredients in the feeding waste.
Degradation of organic matter in the absence of oxygen can be coupled to the reduction of alternative electron acceptors following a certain sequence that appears to be determined by the respective redox potentials. Molecular oxygen (O2/H2O Eh = + 810 mV; Eh values calculated for pH 7.0) is followed by nitrate (NO3– /NO2– Eh = + 430 mV), manganese(IV) oxide (MnO2/Mn2+ Eh = + 400 mV), iron(III) hydroxides (FeOOH/Fe2+ Eh = + 150 mV), sulfate (SO42–/HS– Eh = – 218 mV), and finally CO2 (CO2/CH4 Eh = – 244 mV), with the release of nitrite, ammonia, dinitrogen, manganese(II) and iron(II) carbonates, sulfides, and finally methane as products (Zehnder and Stumm, 1988). Reduction of these acceptors with electrons from organic matter (average redox potential for glucose → 6 CO2 is – 0.434 V; calculated after data of Thauer et al., 1977) provides metabolic energy in the mentioned sequence. Thus, the energy yields of the various anaerobes mentioned differ, and the availability of either highor low-potential electron acceptors may also influence the biochemistry of anaerobic degradation processes.
Limits of anaerobic degradation become obvious with those organic compounds that accumulate in anoxic sediments or that persist in anoxic soil compartments contaminated with mineral oil or other rather recalcitrant compounds. Mineral oil consists mainly of aliphatic and aromatic hydrocarbons which, in the presence of molecular oxygen, are attacked biochemically through oxygenase reactions, which introduce molecular oxygen into the respective molecule (Lengeler et al., 1999; see also Chapter 7). Oxygenase reactions cannot be employed in the absence of oxygen, and, in particular, compounds that require oxygenases for aerobic breakdown might resist degradation under anoxic conditions. Alternatives usually exist in the anoxic world that also allow oxygen-independent degradation of such compounds.
Oxygen is not always advantageous in degradation processes. Oxygenases introduce hydroxyl groups into aromatics, and further oxygen may cause formation of phenol radicals that initiate uncontrolled polymerization and condensation to polymeric derivatives, similar to humic compounds in soil, which are very difficult to degrade further, whether anaerobically or aerobically. Therefore, anaerobic degradation processes may be used for treatment of specific wastewaters rich in phenolic compounds, e.g., from the chemical industry, to avoid formation of unwanted side

8.2 Key Reactions in Anaerobic Degradation of Certain Organic Compounds 231
products such as condensed polyphenols. In other situations, aerobic treatment may cause technical problems, e.g., by extensive foam formation during aerobic treatment of surface-active compounds such as tensides. Thus, knowledge of the limits and principles of anaerobic degradation processes under the various conditions prevailing in natural habitats might help to design suitable alternative techniques for cleanup of contaminated soils or for treatment of specific wastewaters that have so far been applied only insufficiently.
The following survey gives an overview of our present knowledge of the limits and principles of anaerobic degradation of organic compounds. The focus is on those compounds that were for long times considered to be stable in the absence of oxygen.
8.2
Key Reactions in Anaerobic Degradation of Certain Organic Compounds
8.2.1
Degradation of Hydrocarbons
Saturated aliphatic hydrocarbons are attacked only slowly in the absence of oxygen, and the first reliable proof of such a process was provided only about eight years ago for a culture of sulfate-reducing bacteria (Aeckersberg et al., 1991). Growth of this culture with hexadecane was very slow, with doubling times of more than one week under optimal conditions. In the meantime, several strains of alkane-oxidizing anaerobes were isolated (Aeckersberg et al., 1998; Rueter et al., 1994), which are specialized for either long-chain (C12–C20) or medium-chain (C6–16) alkanes and use either sulfate or nitrate as electron acceptor.
Insight into the biochemistry of alkane activation in the absence of oxygen has been obtained only recently. The initial activation is basically similar to the corresponding reaction involved in anaerobic oxidation of toluene (see Section 8.2.6.8): the hydrocarbon is added with its subterminal carbon atom to fumarate through a radical reaction, to form an alkyl succinate derivative (Rabus et al., 2001). This strategy is used in a basically similar manner by nitrate-reducing and sulfate-reducing bacteria (Wilkes et al., 2002). In either case, anaerobic hydrocarbon degradation is very slow, but may play a role in, e.g., natural attenuation of soil sites polluted with petroleum or diesel fuel.
A special example, although not of technical interest, is the anaerobic degradation of methane, e.g., with sulfate as electron acceptor, a process that is of major importance in global carbon transformations. No bacterium that catalyzes this reaction has been isolated so far, although it is thermodynamically feasible (see Schink, 1997). Recent evidence has shown that this reaction is most probably carried out by archaea similar to methanogens, which operate methane formation in the backwards reaction, most often in syntrophic association with sulfate-reducing partner organisms (Boetius et al., 2000). Although this concept was suggested many years ago (Zehnder and Brock, 1980), experimental evidence has been obtained only re-
