
Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdfState of the art developments in immobilised yeast technology for brewing
5. Flavour development and control in immobilised yeast systems
Following the well-known work of Jones and Pierce [14] of the mid 1960's when sequential removal of amino acids from wort was realised, numerous studies have been undertaken to understand the complex interaction of amino acid permeases in order to control the formation of flavour active compounds in brewery fermentations. Amino acid metabolism is obviously critical to beer quality being closely related to the production of vicinal diketones, hydrogen sulphide and higher alcohols [22]. Packed bed fermentations using alginate immobilised lager yeast have been shown to be associated with severe limitations at the levels of amino acid uptake [6], fermentation performance [35], oxygen transfer [17], synthesis of membrane lipids [17] and formation of higher alcohols and esters [35].
These problems were overcome by using the Kirin two-stage free/immobilised cell system [28] or by taking advantage of the superior mass transfer capabilities of fluidised bed [4;6] and gas-lift draft tube fermenters or multichannel loop reactor systems. Thus, in terms of beer quality and fermentation efficiency, optimal mixing will have to be a primary concern in future work on immobilised system design.
A factor common to all high rate processes for primary fermentation is to achieve rapid and efficient removal of vicinal diketones and precursors. The formation of vicinal diketones has been well described, it results from the oxidative decarboxylation of excess oc-acetohydroxy acids leaked from the isoleucine-valine biosynthesis pathway.
The industrial exploitation of genetically modified yeast strains to overcome diacetyl problems is a possible way in which the brewing industry could move today [24;36]. It is, however, most likely that application of recombinant DNA technology to brewer's yeast will be delayed by regulatory requirements and above all by the exclusive rule imposed by tradition and consumer consideration.
In the short-term, greater promise for rapid and complete removal of vicinal diketones may lie with immobilised cell technology. Conversion of a-acetohydroxy acids to vicinal diketones being the rate limiting step, effective control of diacetyl and 2,3 pentanedione levels in the finished beer may be expected by increasing the rate of chemical decarboxylation. This may be achieved by heating the beer after yeast separation. It has been shown that 7 minutes at 90°C are required for complete conversion of oc-acetolactate [31]. Interestingly, under strictly anaerobic conditions, the reaction proceeds directly to acetoin [12].
In this connection, the two alternative approaches which have recently be presented at the EEC congress held in Maastricht in 1997 are the rapid conversion of acetolactate into acetoin using either encapsulated cc-acetolactate decarboxylase [9] or aluminiumsilicate zeolite as a catalyst [3]. In terms of process economics it is clear that, if such treatments prove to be effective, lower cost alternatives to the two-stage heat/cell immobilisation processes could become a reality.
Pajunen et al. [33] have described a commercially used maturation system employing immobilised yeast on granular-derivatised cellulose (DEAE cellulose) material spezyme ® produced by Cultor (Finland). Sintered glass and silicon carbide carriers have also been successfully used for continuous maturation with immobilised yeast [4].
261

C.A. Masschelein and J. Vandenbussche
It has been reported that diacetylreductase has a much higher affinity for diacetyl than alcoholdeshydrogenase which is preferentially reducing acetoin [37]. It is important to note, however, that coenzyme regeneration is a prerequisite for optimal functioning of these reaction sequences. Therefore, immobilised cells must contain intact coenzyme regeneration systems so that high activity can be maintained in the process stream. The long-term stability of the diacetyl reducing capacity of immobilised resting cells highlights the many operational advantages of using mild immobilisation methods. An almost similar situation exists in the fast flowing immobilised yeast systems used for the production of low-and alcohol-free beers. Indeed, fast and effective regeneration of
N ADH and NADPH has been found critical for the conversation of the flavour-potent wort aldehydes to the low flavour-intensive alcohols [8;38].
6. Technological potential of options for immobilised yeast application in the brewing industry
The immobilisation of yeast cells for successful application in brewing implicates the retention of whole catalytic cells within a bioreactor. In order to be a viable alternative to traditional free cell fermentation and maturation systems, immobilised cells must have considerably long working lifetimes, characteristically measured in weeks or months. Mass transfer limitations of substrate into and products out of the immobilised cells and associated matrix are of critical interest [23]. Anticipated criteria for the commercial feasibility of using immobilised cell systems are presented in table 1.
This section describes recent advances in immobilised cell technology and the current commercial options practically applicable to the brewing process for continuous fermentation and/or maturation as well as for the production of malt beverages with defined organoleptic/analytical spectra.
For detailed discussions of immobilised yeast and applications in the brewing industry a comprehensive review has recently be presented by Mensour et al. [27].
262

State of the art developments in immobilised yeast technology for brewing
6.1. IMMOBILISED PRIMARY FERMENTATION
The reactors commonly used for continuous primary fermentation include packed bed, gas lift draft tube and loop reactors.
6.1.1. Packed bed reactor systems
KIRIN BREWERY Co., Ltd (Japan) developed a two stage free/immobilised process witch employs a continuous stirred tank reactor (CSTR) for the first stage and a packed bed reactor for the second stage (figure 2) [13].
Their objective in initiating this technology was to convert wort into beer by a series of steps characterised by cyclic variations in yeast growth and thus to develop a process which resembles the conventional free cell batch fermentation with respect to the metabolic regulatory mechanisms involved in flavour formation.
The free cell chemostat is operated at 13°C. with continuous air sparging (0.017 v.v.m.) in order to achieve a final extract of 8%. The partly fermented beer is centrifuged (less than  cells/ml) and fed to the PBR consisting of a cylindro-conical fermenter with cooling jackets. Bioceramic porous beads with a central pore size of 10-
cells/ml) and fed to the PBR consisting of a cylindro-conical fermenter with cooling jackets. Bioceramic porous beads with a central pore size of 10-
20 urn, a bulk density of  and a surface area of
and a surface area of 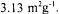 The void volume of the reactor is 40% (vol/vol). The flow rate was controlled to maintain the residual extract between 1.8 and 2.5%. The 20 L pilot plant was scaled up to 5 HL in 1989 and finally to 100 HL in 1991. Major problems encountered with the larger system were operational difficulties related to temperature control and fluid channelling and finally higher capital and operating costs than expected.
The void volume of the reactor is 40% (vol/vol). The flow rate was controlled to maintain the residual extract between 1.8 and 2.5%. The 20 L pilot plant was scaled up to 5 HL in 1989 and finally to 100 HL in 1991. Major problems encountered with the larger system were operational difficulties related to temperature control and fluid channelling and finally higher capital and operating costs than expected.
263
C.A. Masschelein and J. Vandenbussche
Nevertheless, it was decided to proceed with the construction of the restaurant brewery
"Boga Boga" located on Saipan Island in the north Mariana Islands. The continuous plant with a maximum daily production volume of 5 HL has been operational since 1992. While the Kirin system may allow some increase in productivity over the conventional batch process, the added complexity resulting from the chemostat and the need for mechanical centrifuge as well as cooling rods in the packed bed fermenter will probably offset this advantage.
The research team of the HARTWALL Brewing C° from Finland in conjunction with the VTT Biotechnology and Food Research group was assessing the potential of applying porous glass beads 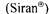 within a two stage packed bed reactor to accomplish the primary fermentation
within a two stage packed bed reactor to accomplish the primary fermentation  Further study is necessary to determine overall process stability before any evaluation of the potential of this technology can be made.
Further study is necessary to determine overall process stability before any evaluation of the potential of this technology can be made.
6.1.2. Gas lift draft tube reactor systems
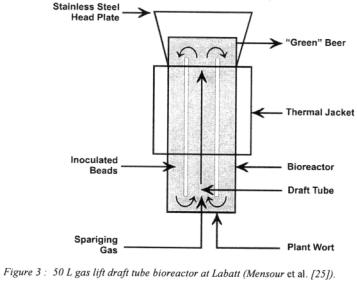
A novel continuous beer fermentation system based on the superior mixing and surface exposure for mass transfer found in gas lift draft tube reactors and the use of Kappa- carrageenan immobilisation carrier has been developed by LABATT Breweries of Canada in collaboration with the Department of Chemical and Biochemical Engineering at the University of Western Ontario (figure 3) [25;26;29].
A 50-L pilot plant was designed and installed for use with the carrageenan beads. To this effect a continuous bead production process based on static mixers was engineered [29]. Beads within a 0.2 to 1.4 mm size range are produced at a maximum throughput of 10 L per hour per static mixer (Canadian Patent 2,133,789,1994).
264
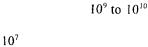
State of the art developments in immobilised yeast technology for brewing
A mixture of air and carbon dioxide is utilised as sparging gas. The proportion of air to 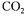 determines the level of yeast growth within the bioreactor as well as the flavour
determines the level of yeast growth within the bioreactor as well as the flavour
profile of the finished product. The yeast concentration |
increases from |
cells |
per ml of gel and remains constant for several months. |
|
|
The level of free cells released from the beads is |
yeast cells per ml |
of liquid |
medium. The residence time for continuous operation at full attenuation is 20-40 hours against a batch fermentation time of 5-6 days. In terms of process evaluation criteria, the gas lift draft tube immobilised yeast bioreactor yields a very high productivity, low energy system. However, a detailed economic analysis is required before the merits of the system can be fully assessed for large-scale industrial applications.
6.1.3. Loop reactor systems
MEURA DELTA, a Belgian R&D engineering company, in association with the research team of CERIA, has developed a two-stage immobilised/free cell loop reactor system for the continuous production of beer [1;2;15;39]. The immobilising carrier is made of sintered silicon carbide particles into a highly porous cylindrical module with 37 internal channels. This 900 mm matrix with outer diameter of 26 mm and 2 mm internal diameter channels has a pore volume of 180 ml.
Advantages for using this particular design are that:
•silicon carbide is an inert material, neutral in taste and food approved with high
mechanical strength and chemical resistance.
•it is suitable for CIP cleaning, re-usable and steam sterilisable.
•it is easy to scale-up by modular assembly.
•it has an ideal preformed shape for optimal mass transfer.
•the open pored structure with a pore size distribution between 40 and 60 um allows
rapid colonisation and efficient gas venting at high 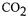 evaluation rates.
evaluation rates.
The first stage consists of a cylindrical vertical vessel in which the silicon carbide matrices are fixed in such a position that the flow of wort is directed within a loop from the bottom of the fermenter through both the internal channels and round about the matrices to the top of the reactor. The circulation is induced by means of a pump and recirculation rate adjusted in order to secure complete mixing and optimal mass transfer.
This particular design has the advantages of both fluidised bed (efficient mixing and gas venting) and a packed bed (mechanically simple) but not the disadvantages of the fluidised bed (high shear with abrasion of carrier material and scale-up problems) nor of the packed bed (plugging, gas flooding and lack of mixing with concomitant diffusion limitations).
An additional advantage of this flow pattern is that wort may be used without any pre-treatment allowing steady-state production over a period of six months. Moreover, cleaning in place is considerably simplified by the possibility of backward and forward flushing. The second stage of the immobilised/free cell reactor system consists of a cylindro-conical vessel. A loop reactor design is used to ensure complete mixing. This is achieved by pumping the fermenting wort taken from the bottom of the fermenter at the top and periphery of the cone.
265

C.A. Masschelein and J. Vandenbussche
Recirculation rate and inlet velocity are adjusted to allow a uniform distribution of cells and substrates inside the reactor. This mechanically simple design permits to maintain sterile operating conditions. The value of a two-stage configuration combining immobilising and free cell reactor systems is that the cells produced in the first stage under constant controlled conditions may be maintained in the growth-limiting environment of the second stage for prolonged periods of time.
The net growth in the second stage will be low but washout does not occur because the first stage continually supplies cells. The experimental set-up is presented in figure 4.
The system was found to be stable and the beer produced had a composition and flavour profile similar to that produced using traditional methods of manufacture. Considering a two-stage immobilised/free cell configuration apparent attenuation values are respectively 35 and 75% using 16°Plato wort and rates of 0.085 1/h.
Thus, 7 HI high gravity beer may be produced on a year basis for a working volume of 5.2 1 so that the volumetric productivity of the system may be rated at 135 Hl/Hl-year or 180 HI after adjusting the original gravity at 12°Plato. The productivity of a plant batch fermentation would be 48 Hl/Hl-year assuming one week to complete attenuation and thus 3.75 times lower as compared to the immobilised/free cell reactor system.
Reduced process times and elimination of lag times and high peak load levels on electricity, steam and other services are clearly advantageous for a fully continuous process. The great advantage intrinsic to immobilised techniques is the availability of high concentrations of catalytic biomass in a controllable form resulting in potentially faster process times. However, immobilised systems have also some important drawbacks, particularly diffusional limitations that have been shown to impact negatively on yeast performance [17]. It can, indeed, be seen from the results in table 2 that using specific sugar consumption rates of 0.034 g/h 109 cells more than 60% of the conversion must be ascribed to the free cells although immobilised cell concentration are about 5 times higher than the free cell concentration (187.106 against 40.106 cells/ml).
266

State of the art developments in immobilised yeast technology for brewing
Consequently, specific sugar consumption rates for the immobilised cells are only
0.0055 against 0.034 for the newly grown free cells. This reduced specific activity is largely due to substrate transport associated limitations which are known to increase with increased cell loading such that maximisation of biomass concentration will be more than offset by a concomitant loss in specific activity. This rate limiting effect is expected to be even more pronounced at low substrate concentrations prevailing in the second stage.
These views find support in the results obtained with a two-stage immobilised system showing that about 85% of the wort sugars fed to the second stage are converted by the free cells. Taking into account both capital cost and catalyst efficiency implications the immobilised/free cell fermenter configuration appears to be the best compromise for industrial application. It is also the most appropriate way to mimic the sequential growth and stationary phase conditions involved in a traditional batch fermentation which are known to be important for flavour development.
6.2. FAST FLOWING IMMOBILISED YEAST SYSTEMS FOR THE PRODUCTION
OF LOW AND ALCOHOL-FREE BEER
Suppression of alcohol formation by arrested batch fermentation is widely accepted as a basic principle for the production of alcohol-free beer. Arrested batch fermentation appears attractive in terms of low capital costs and operating simplicity. It fails when rated on product quality and flavour consistency. Moreover, beers thus produced are often characterised by an undesirable wort taste and aroma.
267

C.A. Masschelein and J. Vandenbussche
The reason for this is that in the early stages of traditional batch fermentation the physiological state of the yeast is varying with the time so that the full potential to reduce the wort aldehydes is never achieved. Optimal steady state conditions can only be maintained by the application of fully continuous processes, preferably combined with the concept of cell immobilisation in order to allow operation beyond the nominal washout flow rate.
Thus in terms of process economics, product quality and operating flexibility small scale high rate immobilised cell systems offer an interesting challenge to traditional batch processing for the production of alcohol-free beer with the desired sensory and analytical profile. The reactors most commonly used for the continuous production of alcohol-free beer with immobilised cells include packed bed, fluidised bed and gas lift loop reactors.
6.2.1 Packed bed reactors
Controlled ethanol production for a low and non-alcohol beer has been successfully achieved by partial fermentation through DEAE cellulose immobilised yeast columns
[41].
A major advantage of this type of carrier is that transport restrictions and diffusional limitations are minimised. This would be an ideal situation, provided that negatively charged wort components or particles are not adversely affecting the binding capacity of brewing yeasts. Accordingly wort treatment and filtration are essential to ensure efficient and controlled fermentation. An industrial scale packed bed reactor is
268

State of the art developments in immobilised yeast technology for brewing
operating at BAVARIA Brewery in the Netherlands for the production of alcohol-free beer. A reactor of 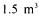 is packed with
is packed with  (400Kg) of Spezyme ® (Cultor) and operated in downflow under strictly anaerobic conditions, low temperature (0-1 °C) relatively high pressure and flowrate of 20HL/h. These conditions have been shown to reduce yeast growth and cellular activity while maintaining a high reducing capacity for wort carbonyl compounds (figure 5).
(400Kg) of Spezyme ® (Cultor) and operated in downflow under strictly anaerobic conditions, low temperature (0-1 °C) relatively high pressure and flowrate of 20HL/h. These conditions have been shown to reduce yeast growth and cellular activity while maintaining a high reducing capacity for wort carbonyl compounds (figure 5).
The system has a production capacity of 150.000 HL beer per annum. If necessary activation steps can be introduced by circulating fresh wort under anaerobic conditions. After 5-7 months the reactor and carrier material are cleaned and sterilised. Other companies in Denmark, Australia and Spain have purchased the CULTOR technology for the production of alcohol-free beer.
6.2.2 Fluidised bed reactors
SCHOTT Engineering has proposed the use of open pored sintered glass beads as carrier material within a fluidised bed reactor for the continuous production of alcoholfree beer [5].
Siran glass beads have been reported to have several advantages for cell immobilisation:
•large active surface area up to 
•controllable pore sizes
•controllable pore volume
•open pore volume of more than 95%
• possibility of varying pore diameter between 10 and 400 urn
•biologically and chemically stable
•easy to clean, reusable and sterilisable with steam
•not compatible
•neutral in taste, food approved
269

C.A. Masschelein and J. Vandenbussche
The system is suited for long term operation and alcohol concentrations in the final product can be adjusted by regulating residence time and/or temperature accordingly.
Brewery BECK & Co have tested a 60 L SCHOTT fluidised bed reactor capable of producing 8HL/day of alcohol-free beer (figure 6) [4]. Wort at 0°C. is used and contact time with the immobilised carrier is adjusted in order to keep the alcohol level below 0.05% by volume.
6.2.3 Gas lift loop reactor
Gas lift multichannel loop reactor systems for the continuous production of low alcohol and alcohol-free beers have been developed by MEURA-DELTA.
The immobilising carrier is constructed of sintered silicon carbide particles into a highly porous cylindrical module with 19 internal channels. This 900 mm matrix with an outer diameter of 25 mm and 2.5 mm internal diameter channels, has a void volume of 30%.
Laboratory, bench and pilot scale bioreactors were constructed and extensively studied under different feeding regimes with single or multi-stage bioreactor configurations allowing the continuous production of alcohol-free beer [38]. A onestage bench scale immobilised cell reactor is presented in figure 7, and has a total holdup volume of 1 litre.
The silicon carbide matrix is installed between two seals creating a closed external chamber. During fermentation a carbon dioxide pressure is build up, draining this external chamber. The excess of 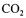 escapes through the porous matrix generating small gas bubbles resulting in an upward flow through the internal channels. The gaslift loop reactor can operate using either natural circulation, or with a circulation pump with an internal circulation rate of 15 renewals per hour for optimal mass transfer. Both
escapes through the porous matrix generating small gas bubbles resulting in an upward flow through the internal channels. The gaslift loop reactor can operate using either natural circulation, or with a circulation pump with an internal circulation rate of 15 renewals per hour for optimal mass transfer. Both
270
