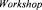Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf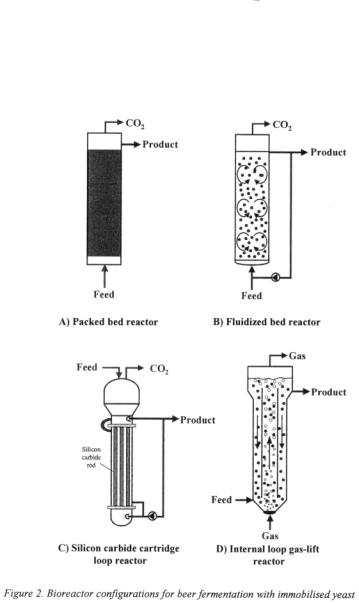
Immobilized yeast bioreactor systems tor brewing recent achievements
2.2. BIOREACTOR DESIGN
Several promising bioreactor concepts for immobilised cell systems have been developed over the last decade related to the choice of the cell carrier (Figure 2).
2.2.1. Packed bed reactor
Packed bed reactors were mostly used for preformed carriers with cells adsorbed on the external surfaces. This type of reactor was widely investigated for beer fermentation since one of the earliest applications of immobilised yeast in this field (Narziss and Hellich, 1971) until recently developed pilot and semi-industrial plants (Kronlof and Virkajarvi, 1999; Inoue, 1995). Packed bad reactors contain cell carriers densely packed
281
Viktor A. Nedovicet al
in a column through which the feed medium (wort) is pumped (Figure 2A). This type of reactor is characterised by a simple design without moving parts providing static conditions for cell cultivation. In this way, cell leakage from carriers with cells adsorbed on external surfaces is minimised. On the other hand, lack of mixing in this type of reactor results in nonuniform feed and temperature distributions, channelling and mass transfer limitations. As a consequence, free amino nitrogen consumption was reportedly low during continuous beer fermentation (Pardonova et 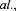 1982; Taidi, 1995).
1982; Taidi, 1995).
One of the ways to avoid channelling and improve heat-transfer recently proposed can be forced circulation of fermenting beer (Andersen et al., 1999; Tata et al., 1999). Nitrogen consumption and beer flavour can be improved in multistage systems based on packed bed reactors. First stage in these systems was designed for aerobic fermentation, which favours amino acid consumption and is followed by packed bed reactors for anaerobic fermentation. In Kirin three-stage reactor system the first stage is carried out in a chemostat, followed by a series of packed bed reactors (Yamauchi and Kashihara,
1996). In a two stage system developed by Kronlof et al. (1996, 1999) aerobic and anaerobic fermentations are carried out consecutively in two packed bed reactors in series. In both multistage systems, final concentration of free amino nitrogen was significantly lowered as compared to one stage packed bed reactor.
2.2.2. Fluidised bed reactor
Fluidised bed reactors were used for carriers with cells adsorbed inside the carrier, made either of glass or ceramics, and carriers with entrapped cells (Shindo et al., 1994b; Umemoto et al., 1998; Breitenbucher and Mistier, 1996; Tata et al., 1999). In this type of reactor the upward flowrate of feed medium is high enough to provide fluidisation of carriers resulting in improved mixing properties and medium distribution as compared to packed bed reactors (Figure 2B). However, in the fluidised state collisions of particles can induce carrier abrasion and damage. In addition, fluidisation of glass and ceramic carriers may require high medium flowrates that could result in high pumping costs and cell leakage. On the other hand, in the case of low density carriers, fluidisation may require low flowrates at which the mass transfer rates could be too low for practical applications.
2.2.3. Silicon 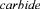 cartridge loop reactor
cartridge loop reactor
Silicon carbide cartridge loop reactor was developed in an attempt to overcome problems inherent to packed and fluidised bed reactors (Van de Winkel, 1995; Masschelein and Andries, 1996; Tata et al., 1999). It consists of silicon carbide multichannel rods (60% void volume) seeded with yeast cells and perfused in parallel with recirculating feed medium (Figure 2C). Cells are grown statically and nutrient supply is provided by medium circulation through the 2-mm diameter channels. The reactor is characterised by simple design, which can be easily scaled up. Disadvantages are still relatively high cost of silicon carbide matrices and lower cell growth and specific productivity in this system as compared to free cells (Masschelein and Andries, 1996).
282
Immobilized yeast bioreactor systems for brewing - recent achievements
2.2.4. Internal loop gas-lift reactor
Our research group (Nedovic et al 1993) successfully introduced three-phase internal loop gas-lift bioreactor in beer fermentation experimental studies. This reactor retains the advantages of fluidised beds, such as high loading of solids and good mass transfer properties and it is particularly suitable for applications with low density carriers (Vunjak et al., 1992,1998). Mixing is established by circulation of liquid and solid phases providing operations at higher liquid flowrates and consequently better mass transfer rates as compared to fluidised bed reactor (Figure 2D). The absence of mechanical agitation creates a relatively low shear environment, which makes these reactors ideally suitable for the application of shear sensitive cells and solid matrices. Low-density alginate and carrageenan gel particles are typically used in three-phase gas-lift reactors as carriers of yeast cells in beer fermentations (Nedovic et al., 1993, 1996, 1997a; Mensour et al., 1996a,b; Vunjak et al., 1998). Other important characteristics of gas-lift bioreactors are simple construction, low risk of contamination, easy adjustment and control of the operational parameters, and simple capacity enlargement (Nedovic et al., 1996,1997b; Mensour et al., 1996a; Mafraetal., 1997).
3. Alginate-gas-lift bioreactor system
Alginate - gas-lift bioreactor system is based on the use of alginate beads as carriers for yeast cells and a three-phase internal loop gas-lift bioreactor. This system was investigated for primary beer fermentation in batch and continuous processes (Nedovic etal., 1996; Nedovic et al., 1997a; Vunjak et al., 1998).
3.1 . ALGINATE MICROBEADS LOADED WITH YEAST CELLS
Alginate has been investigated for a long time for yeast cell immobilisation and beer fermentation. However, several major problems restricted its wider use. Weak mechanical properties make alginate unsuitable for use in packed bed reactors. In addition, only systems for lab scale production of alginate beads with diameters 2 - 4 mm were available. Such beads showed significant diffusion limitations, which reduced cell growth and activity.
Installation of an internal loop gas lift bioreactor with alginate beads for beer fermentation provided new interest for this type of yeast carrier (Nedovic et al., 1993). In parallel, several promising techniques and systems were developed for large scale production of gel-type polymer microbeads over the last decade (Poncelet et al., 1997; Brandenberger and Widmer, 1998; Dulieu et al., 1999; Prttsse et al., 1999). Reduction of bead diameter significantly decreased internal mass transfer limitations.
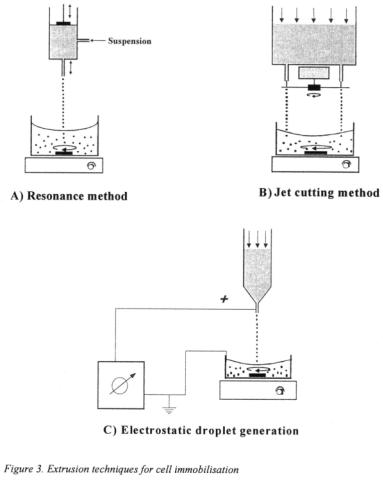
In the case of alginate, microbeads are produced by extrusion of sodium alginate suspension of yeast cells into calcium chloride solution. Sodium ions are immediately replaced with calcium ions and gel beads are formed and solidified due to calcium alginate insolubility in water. Three extrusion techniques gained special attention (Figure 3).
283
Viktor A. Nedovic et a!
Resonance method uses vibration applied at a constant frequency to liquid jet resulting in jet break-up into small uniform droplets in the range of 0.2 to 1.5 mm in diameter
(Figure 3A). Vibration can be applied to the nozzle or to the liquid reservoir. This method can be easily scaled-up and systems for laboratory and pilot plant scales are currently commercially available (Marison et al., 1997; Brandenberger and Widmer, 1998).
Jet cutting method uses mechanical forces to break-up a liquid jet by rotating cutting wires (Figure 3B). Jet is cut into cylindrical segments that attain spherical shape with diameters in the range 0.2 to 2 mm while falling into hardening solution. This technique is commercially available for alginate bead production at industrial scale (Priisse et al.,
1998; Priisse et al., 1999; Wittlich et al., 1999-2000).
284
Immobilized yeast bioreactor systems for brewing – recent achievements
Electrostatic droplet generation uses electrostatic forces to disrupt a liquid surface at the capillary/needle tip forming a charged stream of small droplets (Figure 3C). In this way the liquid is exposed to an electric field, inducing the electrical charge on the liquid surface and a repulsive outside-directed force. This leads to production of uniform small-diameter microbeads down to 0.20 mm in diameter (Bugarski et al., 1994;
Goosen et al., 1997; Melvik et al., 1999; Nedovic et at., 1999b). Presently only a lab scale device with one needle and a power supply of 0-10 kV is commercially available
(Melvik et al., 1999) although scale-up of this system in multi-needle devices with 10 or 20 needles were made recently by several research groups (Gaserad, 1998; Bugarski et al., 1994).
All these techniques provide controlled production of alginate microbeads of 0.2 - 1 mm in diameter with narrow size distributions. Optimal bead diameter for yeast cell immobilisation and application in beer fermentation has yet to be determined. In alginate beads with diameters of about 3 mm cell distribution was nonuniform with dense aggregates close to the surface (around 0.25 mm in depth) implying nutrient limitations (Nedovic, 1999a). Consistently, cell growth in these beads in early phases of cultivation expressed exponential kinetics common for free yeast cells while at later times cell growth was reduced as compared to free cell systems. Alginate microbeads with diameters in the range 0.5 to 1 mm have provided high cell growth without apparent nutrient limitations in a short term cultivation (Nedovic et al., 2000b). Applications of even smaller microbeads may be limited by increased cell leakage.
3.2. INTERNAL LOOP GAS-LIFT BIOREACTOR
Internal loop gas-lift reactor consists of a column with a coaxially placed internal tube.
Liquid phase can be batch or continually supplied usually at the bottom of the column. Solid phase consists of beads suspended in the liquid phase. Gas phase is generally introduced at the bottom of the column into the tube that results in different bulk densities in the tube and the annular region and produces liquid and solid circulation around the tube. The tube is called riser since it contains gas-liquid-solid upflow while the annular region is called "downcomer" containing mainly liquid and solids downflow. For beer fermentation gas phase can be nitrogen or mixture of 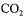 and air, liquid phase is plant wort of 11 - 12 % extract and solid phase are yeast carriers, comprising 1 5 - 4 0 % v/v of rector volume (Nedovic et al., 1993, 1996, 1997a; Mensour et al., 1996a,b; Pilkington et al., 1999). Internal loop gas-lift reactor provides easy temperature control by outer thermal jackets. Liquid and solid circulation may induce increased foaming which is usually solved by addition of antifoaming agents.
and air, liquid phase is plant wort of 11 - 12 % extract and solid phase are yeast carriers, comprising 1 5 - 4 0 % v/v of rector volume (Nedovic et al., 1993, 1996, 1997a; Mensour et al., 1996a,b; Pilkington et al., 1999). Internal loop gas-lift reactor provides easy temperature control by outer thermal jackets. Liquid and solid circulation may induce increased foaming which is usually solved by addition of antifoaming agents.
3.3. BEER FERMENTATION IN ALGINATE-GAS-LIFT BIOREACTOR SYSTEM
Alginate - gas-lift bioreactor system (Figure 4) was successfully applied for batch and continuous primary beer fermentation for up to 3 months at laboratory scale (Nedovic et al., 1993, 1996, 1997a; Vunjak et al., 1998). Alginate beads were shown in repeated batch fermentations to be suitable carriers for yeast cells. Beads with diameters in the range 1 - 2.5 mm loaded with brewing yeast (Saccharomyces uvarum) at concentration
285
Viktor A. Nedovic et al
of about 2xl09 cells/ml showed no significant change in cell activity and viability over a 3 month period (Nedovic et al., 1997a).
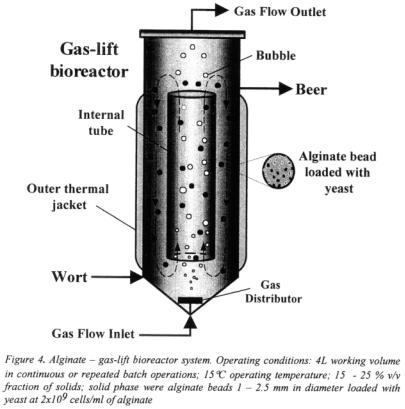
Alginate - gas-lift bioreactor system provided significant fermentation time reduction as compared to traditional beer fermentation, which requires about 7 days (Figure 5).
Primary beer fermentation took 12 to 18 h, depending on operating conditions and desired beer attenuation. The observed reduction of fermentation time can be attributed to high cell loading and efficient bulk mixing in the liquid phase which resulted in rapid mass transfer between the liquid and cell carriers.
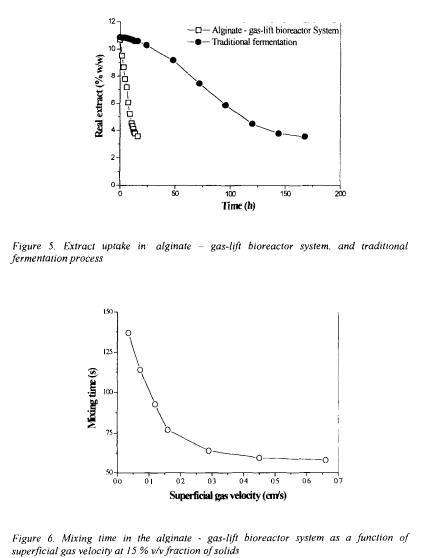
High degree of mixing in internal loop gas-lift reactors was shown to be a consequence of high recirculation of liquid and solid phases in a riser-downcomer part and liquid backmixing in the upper section of the reactor (Obradovic et al., 1994; Nedovic et al., 1997b; Vunjak et al., 1998). Flow in riser and downcomer was described as close to plug flow with low degree of mixing while it was considerably turbulent in the upper section (above the riser) where gas bubbles are disengaged and liquid flow direction is reverted from riser to downcomer. Overall mixing properties of the reactor were found to be mainly determined by superficial gas velocity which was shown to govern both circulation rate and flow pattern (Vunjak et al., 1992; Obradovic et al.,
286
Immobilized yeast bioreactor systems for brewing – recent achievements
1994; Nedovic et al., 1997b). As the superficial gas velocity increased, mixing time rapidly decreased to a certain limit after which no further decrease was observed (Figure 6). This limiting superficial gas velocity corresponds to transition of flow regime where gas starts recirculating together with liquid and solid phases at approximately constant circulation rate.
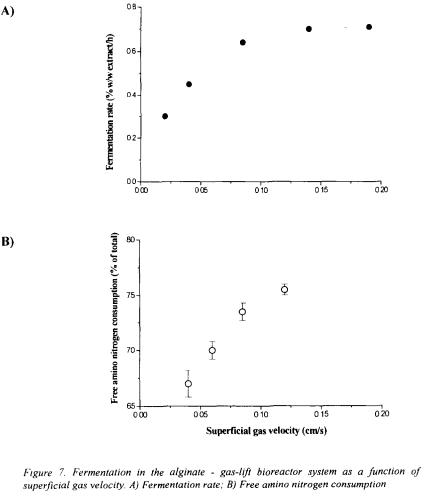
Superficial gas velocity determined also the fermentation rate and free amino nitrogen utilisation, which are dependent on mixing and increased as the superficial gas velocity increased (Figure 7). At superficial gas velocities of about 0.15 cm/s the fermentation
287
Viktor A. Nedovic et al
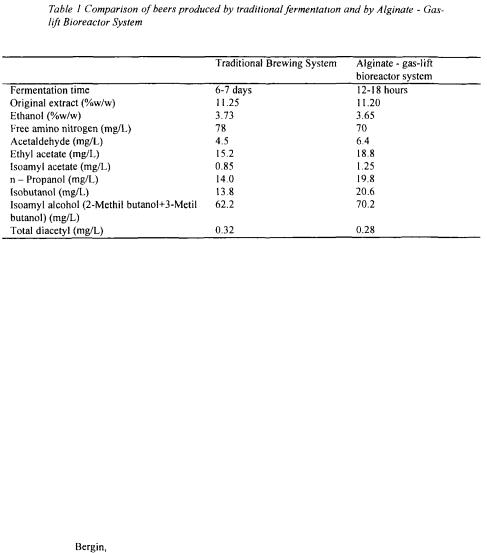
rate reached a limiting value of 0.72 %w/w/h of extract indicating efficient external mass transfer and minimal diffusion boundary layer (Figure 7A). At these conditions, improved amino nitrogen consumption (Figure 7B) resulted in formation of higher alcohols in concentrations typical for lager type beer (Table I). The average concentrations of free amino acids and various flavour-active compounds (e.g. higher alcohols, acetate esters, acetaldehyde, total diacetyl) were comparable with those in beer produced by traditional fermentation (Table 1). Final beer produced in the alginate - gas-lift bioreactor system had desired sensory and analytical profile and could not be distinguished from beer produced by traditional process.
288
Immobilized yeast bioreactor systems for brewing - recent achievements
4. Conclusion
Immobilised cell technology offers favourable solutions for brewing industry. Main advantages are significant reduction of time and cost of beer fermentation. Several attractive concepts for yeast cell immobilisation and bioreactor designs for primary beer fermentation have been developed over the last decade. Mostly used systems are based on yeast cells adsorbed to preformed carriers or entrapped in gel matrices, and cultivated in packed and fluidised bed reactors. Internal loop gas-lift bioreactor with alginate beads loaded with yeast cells is another promising approach to process of main beer fermentation. This system has shown excellent results in repeated batch and
 beer fermentations over up to 3 month period. During this period alginate beads loaded with yeast have shown unchangeable activity and cell viability. Fermentation time was reduced below 1 day and final beers had desired sensory and analytical profiles.
beer fermentations over up to 3 month period. During this period alginate beads loaded with yeast have shown unchangeable activity and cell viability. Fermentation time was reduced below 1 day and final beers had desired sensory and analytical profiles.
References
Andersen, K., |
J., Ranta, B., and Viljava, T. (1999) New process for continuous fermentation of beer, |
in Proc. 27th Congr. Eur. Brew. Conv., Cannes, The European Brewery Convention, Zoeterwoude, pp.
771-779
Brandenberger, H. and Widmer F. (1998) A new multinozzle encapsulation/immobilisation system to produce uniform beads of alginate, J. Biotechnol. 63, 73-80.
Breitenbucher, K. and Mistier, M. (1996) Fluidised bed fermenters for the continuous production of nonalcoholic beer with open-pore sintered glass carriers, in Immobilised yeast applications in the brewing industry, Monograph XXIV, Verlag Hans Carl Getranke-Fachverlag, Nurnberg, pp. 77-88
Bugarski, B., Qiangliang, L., Goosen, M.F.A., Poncelet, D., Neufeld, R.J. and Vunjak, G. (1994) Electrostatic droplet generation: Mechanism of polymer droplet formation, AlChEJ. 40, 1026-1031.
Curin, J., Pardonova, B., Polednikova, M., Sedova, H., and Kahler, M. (1987) Beer production with immobilised yeast, in Proc. 21st Congr. Eur. Brew. Conv., Madrid, IRL Press, Oxford, pp. 433-439
Divies C, Cachon R., Cavin J.-F., and Prevost H. (1994) Immobilised cell technology in wine production, Crit.
Rev. Biotechnol. 14(2), 135-153.
289


 O. (1998) Microcapsules of alginate-chitosan: A study of capsule formation and functional properties,
O. (1998) Microcapsules of alginate-chitosan: A study of capsule formation and functional properties,