
Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf
State of the art developments in immobilised yeast technology for brewing
the porous matrix and down-comer are equipped with cooling jackets for operating at steady-state temperature regimes. The scale up capability was evaluated under industrial conditions for over 12 months. Single stage and two-stage modular pilot scale reactors with 7 or 19 matrices and up to 1.2 hi reactor volume were operative for 4 months without any contamination when strict sterile wort feeding was maintained.
The main objectives were the production of an alcohol-free beer with specifications of 0.05, 0.1 or 0.5% alcohol by volume and sufficient removal of wort carbonyl compounds. Results indicate that the residence time appeared to be a major factor in determining residual aldehyde levels. In contrast to the ethanol production, aldehyde reduction was not additive in the two stages. An average of 85% of the total reduction occurred in the first stage. The reduction in the second stage is much lower as a result of the lower residual aldehyde concentration flowing through the second stage.
From these experiments it was concluded that the scaling-up is reproducible and predictable. Only one stage is necessary for alcohol-free beer production at an optimal temperature of 10°C with minimal aeration levels below 1 mg dissolved oxygen/1.
6.3. IMMOBILISED YEAST SYSTEMS FOR CONTINUOUS FLAVOUR
MATURATION OF BEER
Packed bed reactors employing immobilised yeast on DEAE cellulose or on open pore sintered glass beads are now commercially used for continuous maturation.
6.3.1. DEAE cellulose carrier (Spezyme®)
The first successful industrial process utilising immobilised yeast in the brewing industry has been developed by the finish Co. CULTOR in association with
SYNEBRYCHOFF brewery and the German engineering firm TUCHENHAGEN. The current status of the continuous maturation process has been described by Pajunen et al.  Their objectives in initiating this technology were increased and more flexible volumetric productivity, as well as reducing storage needs. The process parameters were
Their objectives in initiating this technology were increased and more flexible volumetric productivity, as well as reducing storage needs. The process parameters were
271
C.A. Masschelein and J. Vandenbussche
focused on the application of immobilised cells to reach final attenuation and sufficiently reduce diacetyl following an unchanged free-cell primary fermentation.
The equipment (figure 8) includes a hermetic centrifuge, a regenerative plate heat exchanger with a variable volume holding cell, immobilised yeast reactors, and a beer cooler. All of the equipment is constructed out of stainless steel, with automatic valves and flow controls.
In this system, residual yeast in the beer at the end of primary fermentation is centrifuged out to prevent the development of utilised yeast flavours during subsequent processing. Removal of yeast cells (less than 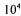 per millilitre) and proteins has been found to be critical with respect to the risk of fouling of the heat exchangers and clogging of the packed beer reactors.
per millilitre) and proteins has been found to be critical with respect to the risk of fouling of the heat exchangers and clogging of the packed beer reactors.
The heat treatment (7 minutes at 90°C) employed by the investigators at
Sinebrychoff enables all the a-acetolactate to be converted to diacetyl. This heat treatment is applied to the beer prior to the immobilised yeast beer downflow to prevent flow channelling frequently recorded in upflow operations.
The contact time of 2 h has proven to be close to the optimum for completing fermentation of the fermentable extract and achieving diacetyl levels below the taste threshold value (0.05 mg/1). The Sinebrychoff maturation system described above has not demonstrated any detectable differences in beer flavour for beer matured using traditional technology. Total lagering time was reduced from 10 to 14 days to 2 to 3 hours. No new synthesis of a-acetolactate was observed in the bioreactor. The only definable process parameter seen to influence beer quality was the length of the heat treatment time. When this time was increased to 20 min. the hydroxymethylfurfural level slightly increased.
An industrial version (1 million hl/y) of this system is now used at the
SYNEBRYCHOFF KERAVA BREWERY in Finland [33]. After the heat treatment there are four reactors with a maximum total flow of 140hl/h, which corresponds to the centrifuge capacity and the time needed for emptying of the fermenters for primary fermentation rapidly. The structure of the immobilised system is modular, allowing 2, 3 or 4 fermenter operations.
Operational advantages of the immobilised yeast system are:
•Short lagtime before steady state
•Standby possible
•Easy and rapid startup
•Immobilisation and regeneration in the reactor
•Modular operation possible
•Flexibility compared with filtration
As a result of the successful industrial exploitation of the two-stage heat/immobilised cell maturation process COMPANHIA CERVEJARIA BRAHMA from Brazil decided in 1993 to test the Cultor Immo-System on an industrial scale with three 10 m3 reactors
[30],
In Brahma’s standard « unitank » process, thermal decarboxylation of acetolactate was achieved immediately after yeast collection and centrifugation to remove the yeast in suspension. Within less than 20 days of the start-up, results allowed to conclude that the
272
State of the art developments in immobilised yeast technology for brewing
Cultor system had the feasibility to complete the secondary fermentation in 2 hours instead of the 12 days required in the traditional process.
Major problems were the difficulty to reach the desired degree of attenuation and the higher  values as compared to the regular products. In contrast foam stability and flavour shelf life were significantly better. Moreover, preliminary results from the taste panel were rather in favour of the immobilised cell treated beer. Unfortunately, further evaluation is not available because of company restructuring and reappraisal of priorities.
values as compared to the regular products. In contrast foam stability and flavour shelf life were significantly better. Moreover, preliminary results from the taste panel were rather in favour of the immobilised cell treated beer. Unfortunately, further evaluation is not available because of company restructuring and reappraisal of priorities.
6.3.2. Sintered glass bead carrier (Siran®)
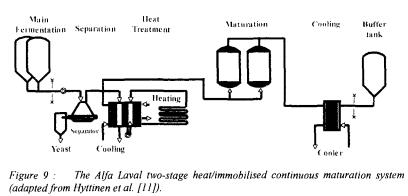
The ALFA LAVAL Co in association with SCHOTT ENGINEERING have developed a maturation process similar to that of Cultor employing the Siran glass beads of Schott within a packed bed reactor.
Laboratory scale tests demonstrated the suitability of using porous glass beads
[11;16]. As a result the HARTWALL BREWING Co from Finland decided to build a 400.000 hi/year maturation plant in 1991 which became operational in 1992 (figure 9).
Two bioreactors with a bed volume of 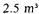 are used. A more even flow, preventing plugging and channelling, was obtained by changing the flow direction from a downwards to an upwards flow. After centrifugation the « Green Beer » was heated to
are used. A more even flow, preventing plugging and channelling, was obtained by changing the flow direction from a downwards to an upwards flow. After centrifugation the « Green Beer » was heated to
80°C for 10 minutes to complete the conversion of a-acetolactate into diacetyl and acetoin. Flavour comparisons have not revealed any differences between the batch and continuous maturation processes.
273
C.A. Masschelein and J. Vandenbussche
7. Concluding remarks
The different advanced immobilised yeast cell bioreactor systems described in this review have been assessed as to their potential for application in the brewing industry. Clearly, many alternatives superior to the conventional batch technology exist.
Reactor concept is an important consideration in immobilised cell system design, although often dictated by the type of application, the support chosen and the desired mass transfer requirements.
The reactors most commonly used for the production of beer with immobilised cells include packed bed, fluidised bed, gas lift draft tube and loop reactors.
Among these only packed bed reactors employing immobilised yeast on DEAE cellulose or porous glass beads have been operated at large scale for continuous maturation as well as for the production of alcohol-free beer and low alcohol beers. In terms of economic evaluation, major advantages are considerable savings in time and plant requirements. Mixed particles reactors including fluidised bed and gas/liquid circulating fermenters have been found more advantageous for primary fermentation. Much progress has been made and many of the processes under development have been advanced to the point of pilot plant testing.
Reduced capital and operation costs are expected. However, these predictions need to be supported by a term of large-scale trials before the potentialities and limitations of continuous immobilised yeast systems can be fully assessed. This is an essential step for allowing economic and quality evaluations to be made on a real basis.
Now the challenge is to view the subject of immobilised cell technology less experimentally and more positively as a permanent feature of future activity in the brewing industry.
References
1.Andries, M., van Beveren, P.C., Coffin, O.& Masschelein, C.A. (1995) Design of a multipurpose immobilised yeast bioreactor for application in the brewing process, Proceedings European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewing Industry, Espoo, Finland, pp. 134-143.
2.Andries, M., van Beveren, P.C., Coffin, O.& Masschelein C.A (1996) Design and application of an immobilised loop bioreactor for continuous beer fermentation, in Immobilised Cells: Basics and Applications (R.H.Wijfels, R.M. Buitelaar, C. Bucke & J. Tramper, eds) Amsterdam: Elsevier Science, pp. 672-678.
3.Andries, M., Derdelinckx, O., lone, K.G., Delvaux, F., van Beveren, P.C., & Masschelein, C.A. (1997) Zeolites as catalysts for the cold and direct conversion of acetolactate into acetoin, Proceedings of the
European Brewery Convention Congress, Maastricht, pp. 477-484.
4.Avaisidis, A., Wandrey, Ch., Eils, H.G. & Katzke, M. (1991) Continuous fermentation of alcohol-free beer with immobilised yeast in fluidised bed reactors, Proceedings of the European Brewery Convention, Lisbon, pp. 569-578.
5.BreitenbUcher, K. & Mistier, M. (1995) Fluidised bed fermenters for the continuous production of nonalcoholic beer with open-pore sintered glass carriers, European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewing Industry, Espoo, Finland, pp. 77-88.
6.Cop, J., Dijon, D., Iserentant, D. & Masschelein, C.A. (1989) Reactor design optimisation with a view
to the improvement of amino acid utilisation and flavour development in calcium alginate brewing yeast fermentations, Proceedings of the European Brewery Convention, Zurich, pp. 315-322.
274
State of the art developments in immobilised yeast technology for brewing
7.Curin, J., Pardonova, B., Polednikova, M., Sedova, H. & Kahler, M. (1987) Beer production with
immobilised yeast, Proceedings of the European Brewery Conventions Congress, Madrid, pp. 433-440.
8.Debourg, A., Laurent, M., Goossens, E., Borremans, E., & Masschelein, C.A. (1994) Wort aldehyde reduction of free and immobilised yeast systems, Journal of the American Society of Brewing Chemists, 52, 100-105.
9.Dulieu, C., Boivin, P., Malanda, M., Dautzenberg, H. & Poncelet, D. (1997) Encapsulation of a- acetolactate dccarboxylase to avoid diacetyl formation, Proceedings of the European Brewery Convention Congress, Maastricht, pp. 455-460.
10.Gronquist, A. Pajunen, E. & Ranta, B. (1989) Secondary fermentation with immobilised yeastindustrial scale, Proceedings of the European Brewery Convention Congress, Zurich, pp339-346.
11. Hyttinen, 1., Kronlof, J. & Hartwall, P. (1995), Use of porous glass at Harwall brewery in the maturation of beer immobilised yeast, European Brewery Convention Symposium: Immobilised Yeast Applications in The Brewing Industry, Espoo, Finland, pp. 55-61.
12.Inoue, T., Murayama, H., Kajino, K., Kamiya, T., Mitsui, S. & Mawatari, M. (1991) Direct spontaneous conversion of acetolactate into non-diacetyl substance in fermenting wort, Proceedings of the European Brewery Convention Congress, Lisbon, pp. 369-376.
13.Inoue, T. (1995) Development of a two-stage immobilised yeast fermentation system for continuous beer brewing, Proceedings of the European Brewery Convention Congress, Brussels, pp. 25-36.
14. Jones, M. & Pierce, J.S. (1964) Absorption of amino acids from wort by yeasts, Journal of the Institute of Brewing, 70, 307-315.
15.Krikilion, Ph., Andries, M., Goffm, 0., Van Beveren, P.C. & Masschelein, C.A. (1993) Optimal matrix and reactor design for high gravity fermentations with immobilised yeast, Proceedings of the European
Brewery Convention Congress, Brussels, pp. 419-426.
16.Kronlof, J., Linko, M. & Pajunen, E. (1995) Primary fermentations with a two-stage packed system – Pilot scale experiences, European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewery Industry, pp. 118-124.
17.Masschelein, C.A., Carlier, A., Ramos-Jeunehomme, C. & Abe, I. (1985) The effect of immobilisation on yeast physiology and beer quality in continuous and discontinuous systems. Proceedings of the European Brewery Convention Congress, Helsinki, pp. 339-346.
18.Masschelein, C.A. & Ramos-Jeunehomme, C. (1985) The potential of alginate immobilised yeast in
brewery fermentations , Proceedings of the European Brewery Convention Congress, Johannesburg,
pp. 392-399.
19.Masschelein, C.A. (1987) New fermentation methods, Proceedings of the European Brewery Convention Congress, Madrid, pp. 209-220,
20.Masschelein, C.A. (1989) Recent and future developments of fermentation technology and fermenter design in brewing, in Biotechnology Applications in Beverage Production, chapter 7, (Cantarelli, C. & Lanzarini, G., Eds) Elsevier Applied Sciences, pp. 41-48.
21. Masschelein, C.A. (1990) Brewing into the 1990s, Proceedings of the 21st Convention of the Institute of Brewing, Australia and New Zealand Section, pp. 6-14.
22. Masschelein, C.A. (1990) Novel fermentation systems: their influence on yeast metabolism and beer flavour, Proceedings of the Third Aviemore Conference on Malting, Brewing and Distilling, pp. 103- 116.
23.Masschelein, C.A., Ryder, D.S. & Simon, J.P. (1994) Immobilised cell technology in beer production,
Critical Reviews in Biotechnology, 14, 155-177.
24.Masschelein, C.A. (1997) A realistic view on the role of research in the brewing industry today,
Journal of the Institute of Brewing, 103, 103-112.
25.Mensour, N., Margartitis, A., Briens, C.L. Pilkington, H. & Russell, I. (1995) Gas lift systems for immobilised cell fermentations, European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewery Industry, Espoo, Finland, pp. 125-132.
26.Mensour, N., Margartitis, A., Briens, C.L. Pilkington, H. & Russell, 1. (1996) Application of
immobilised cell fermentations, in Immobilised Cells: Basics and Applications (R.H. Wijfels, R.M. Buitelaar, C., Bucke & J. Tramper Eds.) Amsterdam: Elsevier Science, pp. 661-671.
27.Mensour, N., Margartitis, A., Briens, C.L. Pilkington, H. & Russell, 1. (1997) New developments in the brewing industry using immobilised yeast cell bioreactor systems, Journal of the Institute of Brewing,
103, 363-370.
275
C.A. Masschelein and J. Vandenbussche
28. Nakanishi, H., Onaka, T., Inoue, T. & Kubo, S. (1985) A new immobilised yeast reactor system for rapid production of beer, Proceedings of the European Brewery Convention Congress, Helsinki, 1985,
pp.331-338.
29.Norton, S., Neufield, R. & Poncelet, D.J.C.M.,  Patent Application, 2, 133, 789, 1994
Patent Application, 2, 133, 789, 1994
30Nothaft, A. (1995) The start-up of an immobilised yeast system for secondary fermentation at Brahma,
European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewery Industry,
F.spoo, Finland, pp. 41-49.
31. E., Makinen, V., & Gisler, R. (1987) Secondary fermentation with immobilised yeast,
E., Makinen, V., & Gisler, R. (1987) Secondary fermentation with immobilised yeast,
Proceedings of the European Brewery Convention Congress, Madrid, pp. 441-448.
32.Pajunen, E.,  A. & Ranta, B. (1991) Immobilised yeast reactor application in continuous secondary fermentation in industrial scale operation, Proceedings of the European Brewery Convention Congress, Lisbon, pp. 361-368.
A. & Ranta, B. (1991) Immobilised yeast reactor application in continuous secondary fermentation in industrial scale operation, Proceedings of the European Brewery Convention Congress, Lisbon, pp. 361-368.
33.Pajunen, E. &  A. (1994) Immobilised yeast fermenters for continuous lager beer maturation, Proceedings of the Institute of Brewing, 23rd Convention Asia Pacific Section, Sidney, pp. 101-103.
A. (1994) Immobilised yeast fermenters for continuous lager beer maturation, Proceedings of the Institute of Brewing, 23rd Convention Asia Pacific Section, Sidney, pp. 101-103.
34.Pilkington, H., Margaritis, A., Mensour, N. & Russell, I. (1998) Fundamentals of immobilised yeast
cells for continuous beer fermentation: a review, Journal of the Institute of Brewing, 104, 19-31.
35.Ryder, D.S. & Masschelein, C.A. (1985) The growth process of brewing yeast and the biotechnological challenge, Journal of the American Society of Brewing Chemists, 43, 66-75.
36.Sane, H., Kondo, K., Fujii, T., Shimuzu, F., Tanaka, J. & Inoue, T., (1987) Fermentation properties of brewer's yeast having a-acetolactate decarboxylase gene, Proceedings of the European Brewery Convention Congress, Madrid, pp. 545-552.
37.Van den Berg, R., Harteveld, P.A. & Martens, F.B. (1983) Diacetyl reducing activity in brewer's yeast,
Proceedings of the European Brewery Convention Congress, London, pp. 497-504.
38.Van de Winkel, L., van Beveren, P.C. & Masschelein, C.A. (1991) The application of an immobilised yeast loop reactor to the continuous production of alcohol-free beer, Proceedings of the European Brewery Convention Congress, Lisbon, pp. 577-584.
39.Van de Winkel, L., Van Beveren, P.C., Borremans, E., Goossens, E. & Masschelein, C.A. (1993) High performance immobilised yeast reactor design for continuous beer fermentation, Proceedings of the European Brewery Convention Congress, Oslo, pp. 307-314.
40.Van de Winkel, L., McMurrough, I., Evers, G., Van Beveren, P.C. & Masschelein, C.A. (1995) Pilotscale evaluation of Silicon carbide immobilised yeast systems for continuous alcohol-free beer production, European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewery
Industry, Espoo, Finland, pp. 90-98.
41.Van Dieren, B. (1995) Yeast metabolism and the production of alcohol-free beer, European Brewery Convention Symposium: Immobilised Yeast Applications in the Brewery Industry, Espoo, Finland, pp. 66-71.
42.White, F.H. & Portno, A.D. (1978) Continuous fermentation by immobilised brewer's yeast, Journal of the institute of Brewing, 74, 228-233.
276
IMMOBILIZED YEAST BIOREACTOR SYSTEMS FOR BREWING – RECENT ACHIEVEMENTS
VIKTOR A. NEDOVIC1, BOJANA  IDA
IDA
LESKOSEK-CUKALOVIC', GORDANA VUNJAK-NOVAKOVIC3
'Institute of Food Technology and Biochemistry, Faculty of Agriculture,
University ofBelgrade, Nemanjina 6, PO Box 127, 11081 BelgradeZemun, Yugoslavia;  of Chemical Engineering, Faculty of Technology, University of Belgrade, Karnegijeva 4, 1 1000 Belgrade, Yugoslavia;
of Chemical Engineering, Faculty of Technology, University of Belgrade, Karnegijeva 4, 1 1000 Belgrade, Yugoslavia;  of Chemical Engineering, MIT, E25-342, 45 Carleton Street, Cambridge, MA 02139, USA
of Chemical Engineering, MIT, E25-342, 45 Carleton Street, Cambridge, MA 02139, USA
During recent years, immobilised yeast technology has gained increasing attention in the brewing industry. As a result of extensive research immobilised yeast technology is nowadays a well established technology for beer maturation and alcohol-free and lowalcohol beer production. However, in primary fermentation the situation is more complex and this process is still under scrutiny on the lab or pilot level. This paper describes several favourable immobilised cell - bioreactor systems for primary beer fermentation with particular emphasis on alginate-yeast microbeads - internal loop gas- lift bioreactor system, introduced into this field by our research group.
1. Immobilised cell systems in biotechnology
Whole cell immobilisation has been defined as "the physical confinement or localisation of intact cells to a certain defined region of space with preservation of some desired catalytic activity" (Karel et al., 1985). Many microorganisms have a capability to adhere to different kinds of surfaces in nature what enables close vicinity to nutrients and food supply. Therefore, we can say that these biological systems are immobilised in their natural state. Furthermore, many biotechnological processes could benefit by immobilisation of biocatalysts. Immobilisation offers many potential advantages over free cell systems, such as higher cell densities and cell loads, increased volumetric productivity, shorter overall reaction times, smaller fermenter sizes which may lower capital costs, reuse of the same biocatalysts for prolonged periods of time due to constant cell regeneration, development of continuous processes which may be performed beyond the nominal washout rate, improved substrate utilisation, reduced risk for microbial contamination, simplified process design, constant product quality, improved tolerance to end products and protection of cells. Above all, immobilised cell technology results in much faster fermentation rates as compared to the existing free
277
M. Hofman and P. Thonart (eds.), Engineering and Manufacturing for Biotechnology, 277–292.
© 2001 Kluwer Academic Publishers. Printed in the Netherlands.
Viktor A. Nedovic et al
cell fermentations. For these reasons there is a growing interest in using immobilised cell systems for different fermentation processes, such as well-known beer (Nedovic et al., 1997a, Pilkington et al., 1998), wine (Divies et al., 1994; Yokotsuka et al., 1997) and cider (Durieux et al., 1998; Nedovic et al., 2000a) fermentations.
2. Applications of immobilised yeast systems in brewing
Application of immobilised cell systems in brewing industry has been investigated for the last twenty years. So far, there are several industrial applications of such systems for the secondary fermentation of beer (Pajunen et al., 1991), and for the production of alcohol-free and low-alcohol beer (Lommi, 1990), but the immobilised cell systems have not been yet successfully applied for primary beer fermentation on industrial level. The main reason for this is wide flavour variation in final beers, which were produced solely by immobilised cells. It was reported that the insufficient free amino nitrogen consumption by immobilised yeast cells, coupled with mass transfer restrictions and reduced cell growth in the immobilised conditions, cause an unbalanced flavour profile of final beer (Hayes et al., 1991). Amino acid metabolism in yeast is closely linked to production of flavour compounds such as vicinal diketones, higher alcohols, esters, organic acids and sulphur compounds. Thus, the way to increase free amino nitrogen consumption and consequently improve beer quality is to increase growth of the cells on one side, and to minimise the internal and external mass transfer resistances in the system on the other.
Internal mass transfer relates to transfer of substrates and products within the carrier, i.e. through the polymeric carrier matrix and dense aggregates of immobilised cells inside the carrier. Internal mass transfer resistances are governed by nature of cell immobilisation, size, texture, and porosity of cell carriers. External mass transfer includes the exchange of nutrients and products between the cell carrier surface and the surrounding medium and it is determined by the bioreactor design and flow pattern. Therefore, two key parameters in immobilised cell systems are the choice of the cell carrier and the bioreactor design.
2.1. CELL CARRIERS AND IMMOBILIZATION METHODS
Main purpose of immobilisation is to retain high cell concentrations within "a certain defined region of space" such as a bioreactor and increase volumetric productivity of a system. Two interrelated directions in research regarding immobilisation can be distinguished: immobilisation techniques and support materials.
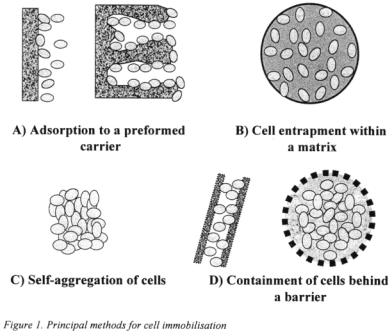
Immobilisation techniques can be divided into four major groups based on physical mechanisms of immobilisation (Pilkington et al., 1998); adsorption to a pre-formed carrier, physical entrapment within a porous matrix, self aggregation in floes and containment of cells behind a barrier (Figure 1). For the immobilisation of yeast cells for beer fermentation, the first two techniques gained most attention.
278
Immobilized yeast bioreactor systems for brewing - recent achievements
2. 1. 1. Adsorption to a pre-formed carrier
The earliest type of cell immobilisation is based on cell adsorption onto external surfaces of solid carriers. Cells can be attached by Van der Waals forces, electrostatic interactions between oppositely charged surfaces, covalent bonding and physical entrapment in the pores. Generally this is the most gentle immobilisation technique as it is carried out by recirculation of cell suspension through a packed bed of carriers, or by mixing a suspension of cells and carriers.
For the adsorption of yeast cells various materials proved suitable, which can be divided in two general types: materials with the yeast cells restricted to the external surfaces only, and materials with pores large enough to allow cell adsorption inside the material (Figure 1A). First group includes DEAE-cellulose and wood chips while the second group includes a variety of glass, ceramic, and synthetic materials. Depending on material, different shapes of cell carriers were applied: non-uniform granules in the case of DEAE-cellulose, spherical porous beads in the case of glass, chitosan and ceramics, porous multichannel rods in the case of silicon carbide, sponge-like porous cubes, and chips or blocks made of wood (Pajunen, et al., 1991; Pajunen, 1996; Lommi, 1990; Inoue, 1995; Yamaushi et al., 1994a,b; Yamauchi and Kashihara, 1996; Masschelein and Andries, 1996; Breitenbucher and Mistier, 1996; Shindo et al., 1994b; Umemoto et al., 1998; Scott and O'Reilly, 1995; Kronlof et al., 1996; Kronlof and Virkajarvi, 1999, Tata et al., 1999).
279
Viktor A. Nedovic et al
In all cases carriers provided mechanically good support and direct contact of cells and substrate, minimising mass transfer limitations. Additional advantage of this immobilisation method can be carrier regeneration and its repeated use. However, in these carriers cells are not protected from the surrounding medium and may be affected by sudden changes in flow, pH, and temperature. The second drawback could be high cell leakage from these carriers coupled with relatively low cell loading in these systems.
2.1.2. Cell entrapment
Cell immobilisation by entrapment is based on low porosity of matrix that at the same time retains cells within the carrier and provides metabolite diffusion (Figure IB). Alginate, kappa-carrageenan and pectate gels in shape of spheres were mostly used as matrix materials for yeast immobilisation (White and Portno, 1978; Pardonova et al.,
1982; Hsu and Bernstein, 1985; Onaka et al., 1985; Curin et al., 1987; Nedovic et al., 1993, 1996, 1997a; Shindo et al., 1994a; Domeny et al., 1996; Mensour et al., 1996a,b; Pilkington et al., 1999).
Two general techniques were developed for the formation of beads loaded with cells: extrusion and emulsification. The extrusion method is based on discharge of cell suspensions into a hardening solution where the gel spheres are formed and hardened. The emulsification method is based on formation of "water in oil" emulsions and solidification of droplets containing suspended cells.
Main advantage of the cell entrapment method is attainment of extremely high cell loading providing high fermentation rates. However, in some cases cell proliferation and activity can be limited by low mass transfer rates within the matrices.
2.1.3. Self-aggregation
Self-aggregation of cells (Figure 1C) can be natural or artificially induced by crosslinking agents. Natural self-aggregation of yeast cells was investigated during
'60ties for continues beer fermentation (Klopper et al., 1965; Stratton et al., 1994) and more recently for beer maturation (Mafra et al., 1997). This technique is based on the use of highly concentrated suspensions of flocculent yeast strains. Although this is the simplest and least expensive immobilisation method it is the most sensitive to the changes in the operating conditions. In addition, there is a high risk of cell wash-out from the system. Another disadvantage could be diffusion limitations in the case of larger floes.
2.1.4. Containment of cells behind a barrier
In this cell immobilisation technique cells are confined to a space bounded by a semipermeable barrier or immobilised within a membrane (Figure ID). There are very little data on yeast immobilisation for beer fermentation by this method. Shindo and Kamimura (1990) reported good mechanical properties and relatively high activity of hollow polyvinylalcohol beads loaded with yeast cells. Still, the procedure for preparation of these beads is relatively complex.
280
