Silvia Andrea Camperi,_Mariano Grasselli and Osvaldo Cascone
4. Conclusions
The high capacity of the membrane cartridge for PE and its excellent hydrodynamic properties allows a very fast fractionation of a commercial pectic enzyme preparation at a low operating pressure. The fraction passing through - containing all the PL activity loaded onto the cartridge - can be used directly to clarify fruit juice without production of methanol.
Acknowledgements
This work was supported by grants from the Universidad de Buenos Aires, the Consejo Nacional de Investigaciones Cientificas y Técnicas de la República Argentina (CONICET) and the Agencia Nacional de Promoción Cientifica y Tecnológica.
M.G. and O.C. are career researchers of the CONICET.
References
Alaña, A., Gabilondo, A., Hernando, F., Moragues, M.D., Dominguez, J.B., Llama, M.J. and Serra, J.I,. (1989) Pectin lyase production by a Penicillium italicum strain, Appl. Envir. Microb. 55, 1612-1616.
Albersheim, P. (1966) Pectinlyase from fungi, in: Neufeld, E.F. and Ginsburg, V. (eds.), Methods in Enzymology, Academic Press, New York, vol. 8, pp.628-631.
Arnold, F.H. (1991) Metal-affinity separations: a new dimension in protein processing, Bio/Technology 9, 151-155
Belew, M., Yip, T.T., Anderson, L. and Porath, J. (1987) Interaction of proteins with immobilised
Quantitation and equilibrium constants by frontal analysis, J. Chromatog. 403, 197-206.
Brandt, S., Goffe, R.A., Kessler, S.B., O’Connor, J.L. and Zale, S.E. (1988) Membrane-based affinity technology for commercial scale purification, Bio/Technology 6, 779-782.
Camperi, S.A, Auday, R.B, Navarro del Cañizo, A.A.and Cascone, O. (1996) Study of variables involved in fungal pectic enzyme fractionation by immobilised metal ion affinity chromatography, Process
Biochem.31, 81-87.
Camperi, S.A., Navarro del Cañizo, A.A., Wolman, F.J., Smolko, E.E., Cascone, O. and Grasselli, M. (1999)
Protein adsorption onto tentacle cation-exchange hollow-fiber membranes, Biotechnol. Prog. 15, 500505.
Chase, H.A. (1984) Prediction of the performance of preparative affinity chromatography, J. Chromatog. 297, 179-202.
Hemdan, E.S., Zhao, Y., Sulkowski, E. and Porath, J. (1989) Surface topography of histidine residues: a facile probe by immobilised metal ion affinity chromatography, Proc Nat. Acad. Sci. USA 86, 18111815.
Kroner, K.H, Krause, S. and Deckwer, W.D. (1992) Cross-flow anwendung von affinitätsmembranen zur primärseparation von proteinen, Bioforum 12, 455-458.
Kubota, N.. Konno, Y., Saito, K., Sugita, K., Watanabe, K., Sugo, T. (1997) Comparison of protein adsorption onto porous hollow-fiber membrane and gel bead-packed bed, J. Chromatog. 782, 159-165.
Mueller-Schulte, D. and Daschek, W. (1995) Application of radiation grafted media for lectin affinity separation and urease immobilisation: a novel approach to tumour therapy and renal disease diagnosis,
Radiat. Phys Chem. 46, 1043-1047.
Navarro del Cañizo, A.A., Hours, R.A., Miranda, M.V. and Cascone, O. (1994) Fractionation of fungal pectic enzymes by immobilised metal ion affinity chromatography, J. Sci. FoodAgric. 64, 527-531.
Porath, J., Carlsson, J., Olsson, I. and Belfrage, G. (1975) Metal chelate affinity chromatography, a new approach to protein fractionation, Nature 258, 598-599.
Rombouts, F.M. and Pilnik, W. (1980) Pectic Enzymes, in: Rose AH (ed.) Economic Microbiology, Academic Press, London, vol. 5, pp. 228-282.


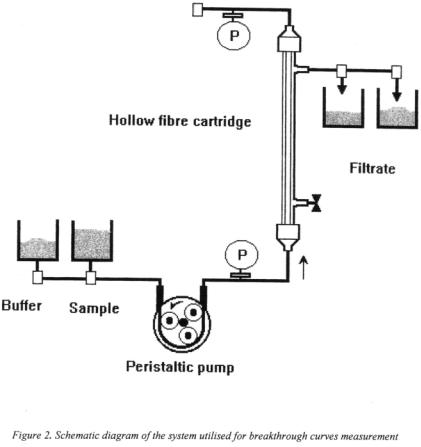
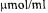 and a pure water SV of 234
and a pure water SV of 234 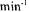 at a
at a 
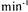 and with an input stream adsorbate concentration of 600.3 U/ml of PE and 289 U/ml of PL. The dynamic capacity of the column under these conditions was 7500 PE U/ml. PL was not adsorbed by the chromatographic membrane.
and with an input stream adsorbate concentration of 600.3 U/ml of PE and 289 U/ml of PL. The dynamic capacity of the column under these conditions was 7500 PE U/ml. PL was not adsorbed by the chromatographic membrane.