Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf
INITIATION, GROWTH AND IMMOBILISATION OF CELL CULTURES OF
TAXUS SPP. FOR PACLITAXEL PRODUCTION
CHI WAI TANG, EMAN ZALAT AND FERDA MAVITUNA
Department of Chemical Engineering, UMIST, PO Box 88, Manchester
M60 IQD, UK.
E-mail: f.mavituna@umist.ac.uk Fax No: 44 161 200 4399
Summary
Paclitaxel (Taxol), a cytotoxic diterpene initially isolated from the bark of Taxus brevifolia (Pacific yew), has been approved for cancer treatment. Since total chemical synthesis is not economical, plant biotechnology can offer an alternative route for the production of this drug. Callus cultures were initiated from different explants of Taxus x media and Taxus cuspidata on various media using different plant growth regulators in different photo-regimes. Several fast growing, white, friable calli lines were established.
Suspension cultures were initiated from these calli and the activities of suspension and immobilised cultures were monitored over 40 days in shake flasks and bioreactors. Cell immobilisation in reticulated polyurethane foam particles showed improved growth. Since the specific paclitaxel yield were the same for both suspended and immobilised cultures, higher biomass concentration in the immobilised cultures led to higher paclitaxel concentration in the medium.
1. Introduction
1 . 1 . PHARMACEUTICALS FROM PLANTS
Plants remain to be a significant source of pharmaceuticals for the treatment of a wide range of human ailments. Natural products isolated from higher plants account for approximately 25% of the prescribed drugs used in “Western” civilisation (Balandrin et al., 1993; Farnsworth and Morris, 1976). In the United States alone, in 1990, this corresponded to a market value of approximately $ 15.5 billion (Principe, 1996). In the developing countries, 85% of the drugs used for healthcare is based on traditional medicine mainly derived from plants (Pezzuto, 1996). This implies that about 80% of the world's population rely on plant-derived natural products for their primary
429
M. Hofman and P. Thonart (eds.), Engineering and Manufacturing for Biotechnology, 429–448. © 2001 Kluwer Academic Publishers. Printed in the Netherlands.

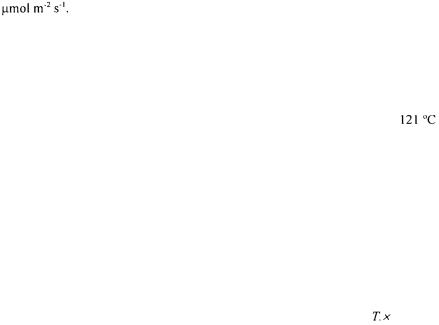


 media
media 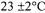
 media (Table 1). In addition to the growth regulators, these basal media were supplemented with 20g/l sucrose and 3.5g/l
media (Table 1). In addition to the growth regulators, these basal media were supplemented with 20g/l sucrose and 3.5g/l foam particles were
foam particles were