Immobilized yeast bioreactor systems for brewing – recent achievements
Nedovic V.A., Leskosek-Cukalovic I., and Vunjak-Novakovic G. (1996) Short-time fermentation of beer in an immobilised yeast air-lift bioreactor, in J. Harvey (ed.), Proc. 24th Conv. Inst. Brew., Singapore, Winetitles, Adelaide, p. 245
Nedovic, V.A., Leskosek-Cukalovic, I., Milosevic, V., and Vunjak-Novakovic, G. (1997a) Flavour formation during beer fermentation with immobilised Saccharomyces cerevisiae in a gas-lift bioreactor, in F.
Godia and D. Poncelet (eds.) Proc. Int. Workshop Bioencapsulation VI: “ From fundamentals to industrial applications” , Barcelona, Spain, T5.3, pp. 1-4
Nedovic, V.A., Pesic, R., Leskosek-Cukalovic, I., Laketic, D., and Vunjak-Novakovic, G (1997b) Analysis of liquid axial dispersion in an internal loop gas-lift bioreactor for beer fermentation with immobilised yeast cells, in M. Olazar and M.J.San Jose (eds.), Proc. II European Conference on FWIDIZATION, The
University of the Basque Country Press Service, Bilbao, pp. 627-635
Nedovic, V.A. (1999a) Immobilised cell systems in beer fermentation, Monograph (in Serbian), Andrejevic
Foundation, Belgrade.
Nedovic, V.A., Trifunovic, O., Pesic, R., Leskosek-Cukalovic, I., Bugarski, B. (1999b) Production of microbeads containing immobilised yeast cells for continuous beer fermentation by electrostatic droplet generation, in G. Skjåk-Braek and D, Poncelet (eds.), Proc. Int. Workshop Bioencapsulation VIII:
“Recent Progress in Research and Technology”, Trondheim, Norway, P-4, pp. 1-5
Nedovic, V.A., Durieux, A., Van Nedervelde, L., Rosseels, P., Vandegans, J., Plaisant, A.-M., and Simon, J.-P. (2000a) Continuous cider fermentation with co-immobilised yeast and Leuconostoc oenos cells,
Em. Microb. Technol. 26, 834-839.
Nedovic, V., Obradovic, B., Leskosek-Cukalovic, I., Trifunovic, O., Pesic, R., and Bugarski, B. (2000b) Electrostatic generation of alginate microbeads loaded with brewing yeast, Submitted to Proc. Biochem.
Obradovic, B., Dudukovic, A., Vunjak-Novakovic, G. (1994) Local and overall mixing characteristics of the Onaka, T., Nakanishi, K., Inoue, T., and Kubo, S. (1985) Beer brewing with immobilised yeast,
Pajunen E., Gronqvist A. and Ranta B. (1991) Immobilised yest reactor application in continuous secondary fermentation in industrial scale operation, in Proc.  Congr. Eur. Brew. Conv., Lisbon, ILR Press, Oxford, pp. 361-368
Congr. Eur. Brew. Conv., Lisbon, ILR Press, Oxford, pp. 361-368
Pajunen, E. (1996) Immobilised yeast lager beer maturation: DEAE-cellulose at Sinebrychoff, in
Immobilised yeast applications in the brewing industry, Monograph XXIV, Verlag Hans Carl Getranke-
Fachverlag, Nurnberg, pp. 24-34
Pardonova, B., Polednikova, M., Sedova, H., Kahler, M., and Ludvik, J. (1982) Biokatalysator fur die bierherstellung, Brauwissenschaft 35, 254-258.
Pilkington H., Margaritis A., Mensour N.A., and Russell I. (1998) Fundamentals of immobilised yeast cells for continuous beer fermentation: A review, J. Inst. Brew. 104, 19-31.
Pilkington, P.H., Margaritis, A., Mensour, N.A., Sobczak, J., Hancock, I., Heggart, H., and Russell, I. (1999)
The use of kappa-carrageenan gel to immobilize lager brewing yeast, in G. Skjak-Brek and D. Poncelet
(eds.), Proc. Int. Workshop Bioencapsulation VIII: "Recent Progress in Research and Technology",
Trondheim, Norway, P-17, pp. 1-4.
Poncelet, D., Dulieu, C., and Neufeld, R.J. (1997) Fundamentals of the dispersion into encapsulation technology, in F. Godia and D. Poncelet (eds.) Proc. Int. Workshop Bioencapsulation VI: "From fundamentals to industrial applications", Barcelona, Spain, T1.1, pp. 1-4
Prusse, U., Bruske, F., Breford, J., and Vorlop, K.-D. (1998) Improvement of the jet cutting method for the preparation of spherical particles from viscous polymer solutions, Chem. Eng. Technol. 21, 153-157.
Prilsse, U., Dalluhn, J., Sonnenburg, J., Breford, J., and Vorlop, K.-D. (1999) Scale-up strategies for bead production by jet cutting, in G. Skjåk-Braek and D. Poncelet (eds.), Proc. Int. Workshop Bioencapsulation VIII: “Recent Progress in Research and Technology”, Trondheim, Norway, O-6, pp.
1-4
Scott, J.A. and O’Reilly A.M. (1995) Use of a flexible sponge matrix to immobilize yeast for beer fermentation, J. Am. Soc. Brew. Chem. 53, 67-71.
Shindo S and Kamimura, M. (1990) Immobilisation of yeast with hollow PVA gel beads, J. Perm. Bioeng. 70, 232-234.
Shindo, S., Sahara H., and Koshino S. (1994a) Suppression of α -acetolactate formation in brewing with immobilised yeast, J. Inst. Brew. 100, 69-72.



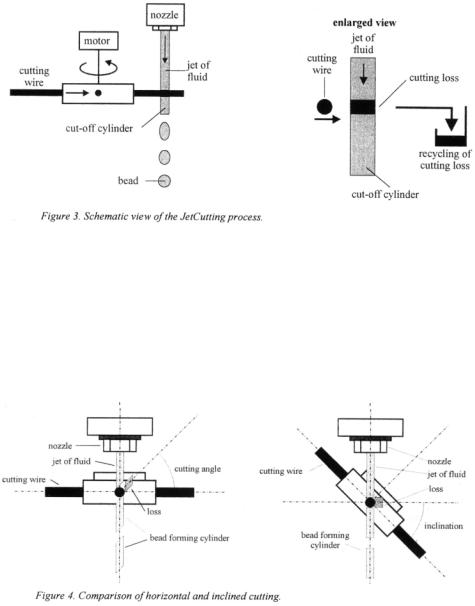

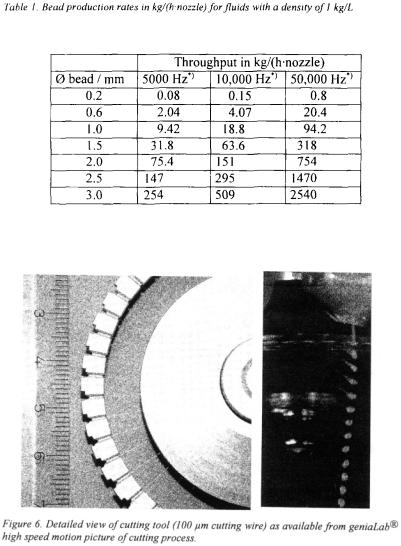

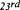
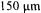 at the moment, the upper limit is about 2.5 to 3 mm (depending on the properties of the fluid).
at the moment, the upper limit is about 2.5 to 3 mm (depending on the properties of the fluid).