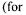Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf
A NEW POLYSACCHARIDE DERIVED FROM PLANT RHIZOSPHERE : PRODUCTION, PURIFICATION AND PHYSICO-CHEMICAL PROPERTIES
CROMPIN J.M., GARNIER T., PAYOT T., DE BAYNAST R.
ARD (Agro-Industry Research and Development), F-51110 Pomacle E-mail: ard@wanadoo.fr Fax : (33) 03.26.05.42.88
Summary
Microbial polysaccharides are of great interest on the market of hydrocolloïds. The development of a new one is to be considered only if its properties are different and complementary of those already existing. It is the case of "Soligel", a new exopolysaccharide produced by Rhizobium sp.
1. Introduction
The most part of industrial polysaccharides are originated from plants (starch, cellulose, gum arabic, pectins, guar gum, locust bean gum ...) and seaweed (agar, carrageenans, alginates). However, their production may be subjected to several hazards (drought, crop failure, war, famine...), causing lack of an assured supply and variations in quality.
Comparatively, microbial polysaccharides offer numerous advantages : their production in bioreactor is controlled and reproducible. They are synthesised by a great diversity of microorganisms, with different compositions and/or structures. These macromolecules still have higher production costs than traditional polysaccharides. In order to compete with the latter, they must have better rheological properties and/or new functions.
For instance, microbial polysaccharides are used as thickening, gelling, flocculating or moisturising agents, in a wide domain of applications : food, agriculture, cosmetic, pharmacy, environment... (Paul et al., 1986 ; Sutherland, 1998).
The strategy of ARD is to valorise European crops and associated by-products. ARD has found potential substrates for production of polysaccharides by using these renewable raw materials. Consequently, the research of a new polysaccharide with original properties has been initiated through an AGRICE program (AGRIculture for Chemistry and Energy), with specific tasks in the areas of microbiology, chemistry and processing.
423
M. Hofman and P. Thonart (eds.). Engineering and Manufacturing for Biotechnology, 423–428.
© 2001 Kluwer Academic Publishers. Printed in the Netherlands.

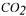 pressure : elaboration of sparkling wines
pressure : elaboration of sparkling wines of beads per day). An economic study undertaken for a plant producing 3,000,000
of beads per day). An economic study undertaken for a plant producing 3,000,000 operating for 220 days. A continuous system has been experimented by Fumi
operating for 220 days. A continuous system has been experimented by Fumi 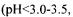
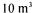 fermentor.
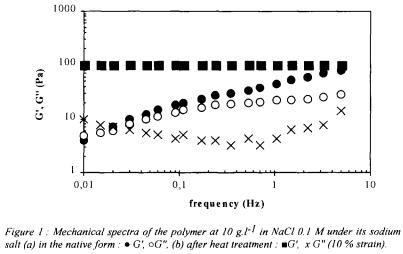
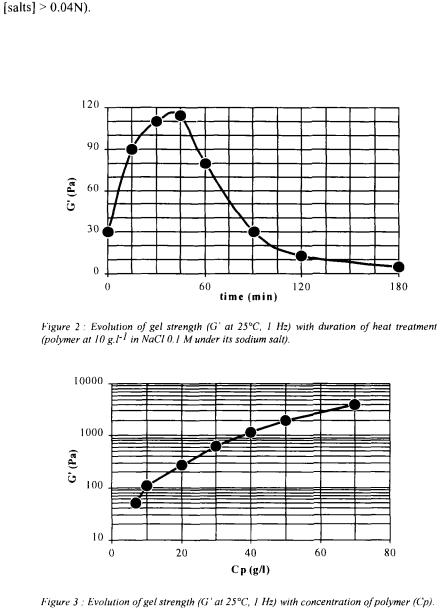
fermentor.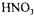 or NaOH. The measurements of the viscoelastic properties of the gels were performed using a rotary rheometer with coneplate measuring heads (TA Instruments, AR1000).
or NaOH. The measurements of the viscoelastic properties of the gels were performed using a rotary rheometer with coneplate measuring heads (TA Instruments, AR1000).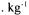 in the broth was observed from the laboratory to the pilot scale, with good conversion coefficient of substrate into exopolysaccharide (50 to 60%). Recent data indicated the possibility of improving the productivity and the conversion coefficient by feeding continuously the fermentor with carbon source. The kinetic data seem to be the same as those of xanthan (Amanullah
in the broth was observed from the laboratory to the pilot scale, with good conversion coefficient of substrate into exopolysaccharide (50 to 60%). Recent data indicated the possibility of improving the productivity and the conversion coefficient by feeding continuously the fermentor with carbon source. The kinetic data seem to be the same as those of xanthan (Amanullah