
Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf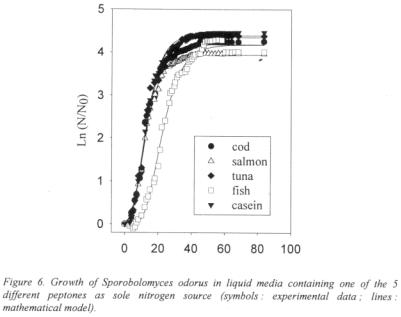
Enzymic solubilisation of proteins from tropical tuna using alcalase
4. Conclusion
The controlled hydrolysis of tuna stomach proteins was a good alternative to upgrade marine by-products from the fisheries industry. The reproducibility of the hydrolysates chromatographic profiles between each run was checked. This outlined the importance of the first step of raw material preparation (heat inactivation) and of using exogenous proteases (Alcalase, for example) for ensuring reproducibility of sample preparation and for obtaining the target peptide size.
The fractionation method presented in this work was based on FLPC gel filtration chromatography using the SUPERDEX HR10/30 column. This method gave a good resolution of peptidic fractions and was useful for the follow up of protein proteolysis and the evaluation of the hydrolysis degree.
As far as cellular growth factors are concerned, the tuna hydrolysate tested exerted a stimulatory effect on the protein synthesis in fibroblast cells and the presence of gastrinlike peptides in the tuna hydrolysate has been detected.
The tuna hydrolysates used as nitrogenous substrate are promising with regards to the preliminary results obtained for the strains studied, and fish peptones should be thoroughly investigated to find industrial applications.
49
Fabienne Guerard et al
References
Adler-Nissen, J. (1982). Limited enzymatic degradation of proteins : a new approach in the industrial application of hydrolases. J. Chem. Tech. Biotechnol. 32, 138-156.
Bautista, J., Hernadez-Pinson, I., Alaiz, M., Parrado, J., Millan, F. (1996). Low molecular weight sunflower protein hydrolysate with low concentration in aromatic amino acids. J. Agric. Food Chem 44, 967
Benjakul, S. & Morrissey, M. T. (1997). Protein hydrolysates from Pacific Whiting solid wastes. J. Agric. Food Chem. 45, 3423-3430.
Cancre, I., Ravallec, R., Van Wormhoudt, A., Stenberg, E., Gildberg, A. & Le Gal, Y. (1999)., Secretagogues and growth factors in fish and crustacean protein hydrolysates. Mar Biotechnol 1 :489494
Carmichael, J., Degraff, W.G., Gazdar, A.F., Minna, J.D. & Mitchell, J.B.(1987). Evaluation of a tetrazolium-based semiautomated colorimetric assay : assessment of chemosensitivity testing. Cancer Res. 47(4), 936-942.
Diniz, F. M. & Martin, A. M. (1998). Influence of process variables on the hydrolysis of shark muscle protein. Food Sci. Technol. Intern. 4, 91-98.
Dufosse, L., De La Broise, D. & Guérard, F. (1997) - Fish protein hydrolysates as nitrogen sources for microbial growth and metabolite production.  Recent Research Developments in Microbiology,
Recent Research Developments in Microbiology,  S.G. PANDALAI
S.G. PANDALAI  Research Signpost, ISBN 81-86481-50-8, pp. 365-381.
Research Signpost, ISBN 81-86481-50-8, pp. 365-381.
F.A.O. (1997). Review of the state of world fishery resources : marine fisheries. FAO Fisheries Circular
920 FIRM/C920
Fouchereau-Peron, M., Duvail, L., Michel, C., Gildberg, A, Batista, I.& Le Gal, Y. (1999) Isolation of an acid fraction from a fish protein hydrolysate with a CGRP-like biological activity. Biotechnol.Appl. Biochem., 29,87-92.87
Guerard, F., Dufossé, L., De La Broise, D. & Binet, A. (2000). Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using Alcalase. Journal of molecular catalysis B :Enzymatic (in press)
Hoyle, N. T. & Merritt, J. H. (1994). Quality of fish protein hydrolysates from Herring (Clupea harengus). J.
Food Science 59, 76-79.
Le Gal Y. & Stenberg E. (1998). Tout est bon dans le poisson, Biofutur, 179
Martin, A. M. & Porter, D. (1995). Studies on the hydrolysis of fish protein by enzymatic treatment. In Food flavours : generation, analysis and process influence., pp. 1395-1404. Edited by G. Charalambous. Amsterdam: Elsevier.
Quaglia, G. B. & Orban, E. (1987). Enzymatic solubilisation of proteins of sardine (Sardina pilchardus) by commercial proteases. J. Sci. Food Agric. 38, 263-269.
Shahidi, F., Han, X. Q. & Synowiecki, J. (1995). Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 53, 285-293.
Acknowledgements
This work was supported by the Commission of the European Communities, Directorate-General XII for Research, Technological Development and Demonstration in the Field of Agriculture and Agro-Industry (FAIR) - FAIR contract CT 97-3097. The authors are grateful to the NOVO company, which generously provided the Alcalase preparation.
50
INFLUENCE OF THE EXPERIMENTAL CONDITIONS ON THE HYDROLYSIS PROCESS IN FISH HYDROLYSATES.
ROZENN RAVALLEC-PLE*, LAURA GILMARTIN**, ALAIN
VAN WORMHOUDT* AND YVES LE GAL*
*Marine Biology-MNHN- 29182 Concarneau, France
**University of Plymouth-Plymouth-Devon PL4 8AA, United Kingdom
Summary
Protein hydrolysates were prepared from cod muscle (Gadus morhua) using commercial Alcalase® in different experimental conditions and were studied in order to determine the influence and the importance of each factors such as pH, temperature and enzyme/substrate ratio on the hydrolysis degree.
1. Introduction
Atlantic cod is an abundant source of waste, particularly due to the filleting process, and has previously been included in Atlantic salmon (Salmo salar) diets (Gildberg et al., 1995). The nutritional value of processing discards of cod has been investigated and has been found to be a sustainable and economically attractive protein feed supply for the aquaculture industry (Shahidi et al. 1991).
It is well known that a high quality fish protein hydrolysate (FPH) can be commercially produced from fish wastes with simple engineering (Chakraborty and Madhavan , 1977) and can be used as animal feed or fertiliser (Venugopal, 1994). Different processes (hydrolysis, autolysis) permitted to generate molecules larger than individual amino-acids that could be of economical interest in the development of aquaculture. A lack of diets adapted to the larval development in fish or crustacean species encourages the research of adequately alimentation. The level of hydrolysis seems to play an important role for the presence of biological peptides related to growth factors or gastrin (Cancre et al., 1999), calcitonin (Fouchereau-Peron et al., 1999) or opioïds (Piot et al., 1992). Gastrin and cholecystokinins are small peptides but growth factors are bigger molecules and we observed that an extensive process is not necessary to obtain interesting biological activities, on the contrary, a high hydrolysis degree
51
M. Hofman and P. Thonart (eds.), Engineering and Manufacturing for Biotechnology, 51–58 . © 2001 Kluwer Academic Publishers. Printed in the Netherlands.
Rozenn Ravallec-Ple, Laura Gilmartin, Alain Van Wormhoudt and Yves Le Gal
correspond to an important reduction in the size of the molecules (Ravallec-Plé et al. 2000).
These preliminary results have shown that extensive hydrolysis is not an advantage important but the reduction of size of the original macromolecules is necessary.
One of the potential use of enzymes for the modification and improvement of protein functionality is trough controlled hydrolysis to assure reproducibility of the process (Quaglia and Orban, 1987b). Some studies showed that different conditions of hydrolysis like time, temperature, and the ratio enzyme/substrate will generate different hydrolysis process (Quaglia and Orban, 1987a).
In this context, the purpose of this work was to study the influence of the process parameters on the hydrolysis of cod stomach by a bacterial endopeptidase with low specificity, the Alcalase®.
2. Materials and methods
2.1. SUBSTRATE
Specimens of cod (Gadus morhua) were kindly provided by the market of Concarneau. Only the muscle was utilised ; this was boiled 20 minutes, blended and kept frozen at - 20°C until it was used.
2.2. ENZYMES
The three enzymes used for the hydrolysis were provided by Novo NORDISK Industri, Denmark. Alcalase® is a serine bacterial endopeptidase (generic name : subtilisin Carlsberg) prepared from a strain of Bacillus licheniformis with a specific activity of 2.4 AU/g (Anson units, Anon, 1988). Neutrase® is an endoprotease produced by a selected strain of Bacillus subtilis with a specific activity of 0.5 AU/g. Protamex® is a Bacillus protease complex developed for hydrolysis of food proteins with a declared activity of 1.5 AU/g. The food-grade enzymes were stored at 5°C until they were used for the hydrolysis experiment. Their optimum activities occur at temperatures between 40°C and 60°C (70°C for Alcalase®) and at pH values between 6 and 10. The deactivation was made at 85°C for 10 minutes (Anon 1988, 1991).
2.3. HYDROLYSIS
Cod muscle (Gadus morhua) hydrolysates were produced by hydrolysis in different experimental conditions of 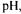 temperature, time and enzyme concentration. The hydrolysis was controlled using the
temperature, time and enzyme concentration. The hydrolysis was controlled using the 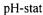 method (Boyce, 1986) by addition of 2N NaOH for 2h on 50g of raw material in 450 ml of distilled water. After deactivation of the enzyme, the hydrolysate was centrifuged at 20000g during 30 minutes and lyophilised to obtain a powder. The hydrolysis degree was calculated using the
method (Boyce, 1986) by addition of 2N NaOH for 2h on 50g of raw material in 450 ml of distilled water. After deactivation of the enzyme, the hydrolysate was centrifuged at 20000g during 30 minutes and lyophilised to obtain a powder. The hydrolysis degree was calculated using the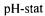 and the trinitrobenzenesulphonic acid (TNBS) methods (Adler-Nissen, 1982; Adler-
and the trinitrobenzenesulphonic acid (TNBS) methods (Adler-Nissen, 1982; Adler-
52

Influence of the experimental conditions on the hydrolysis process in fish hydrolysates.
Nissen, 1979). The protein content was determined by the Kjeldahl nitrogen analysis (Lynch et al., 1998).
2.4. STATISTICAL ANALYSIS
Degree of hydrolysis (DH) is generally used as proteolysis factor when the  method was used. The
method was used. The 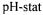 reaction allows the estimation of DH based on the consumption of alkali to maintain a constant
reaction allows the estimation of DH based on the consumption of alkali to maintain a constant 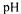 at desired value. Response Surface methodology (Statgraphic 2.0) was employed to determine the influence and the importance of the different factors such as
at desired value. Response Surface methodology (Statgraphic 2.0) was employed to determine the influence and the importance of the different factors such as 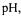 temperature and ratio enzyme/substrate on the degree of hydrolysis (DH) of the cod muscle by the enzyme Alcalase® and to optimise them. The following table details the experimental design and the average results of three experiments at each point. Using this data contour plots were drawn for each of the factors.
temperature and ratio enzyme/substrate on the degree of hydrolysis (DH) of the cod muscle by the enzyme Alcalase® and to optimise them. The following table details the experimental design and the average results of three experiments at each point. Using this data contour plots were drawn for each of the factors.
53
Rozenn Ravallec-Ple, Laura Gilmartin, Alain Van Wormhoudt and Yves Le Gal
2.5. FPLC CHROMATOGRAPHY
Hydrolysates (1mg/ml) were further analysed by gel filtration on a Superdex Peptide HR 10/30 column (1×30 cm) using acetonitrile (30%) in water with TFA (0.1%) as eluent and a flow rate of 0.5 ml/min according to Guerard et al.(2000).
The chromatography was monitored by measuring the absorbance at 220 nm. Column calibration was performed using Ribonuclease A (13700 Da), Aprotinin (6500 Da), Angiotensin I (1296 DA), Bradykinin (1060 Da), Angiotensin III (931 Da), Hexaglycine (360 Da), Tetraglycine (246 Da), Triglycine (189 Da) and Diglycine (132 Da).
3.Results and discussion
3.1.EFFECT OF THE ENZYME ON THE DEGREE OF HYDROLYSIS
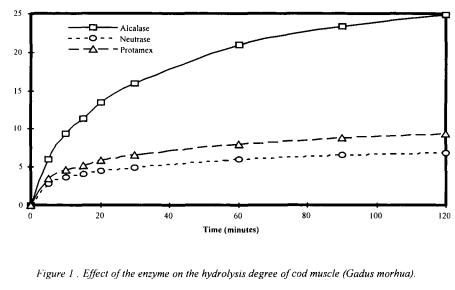
The DH values after the 2 hours hydrolysis of cod proteins by the three different enzymes were reported figure 1.
Enzymes were used at a concentration of 1% (volume/weight of raw material) at specific  and temperature (Alcalase® : 8, 40°C - Neutrase® and Protamex® : 7, 40°C). The highest DH was obtained with Alcalase® (24.85%), the lowest with
and temperature (Alcalase® : 8, 40°C - Neutrase® and Protamex® : 7, 40°C). The highest DH was obtained with Alcalase® (24.85%), the lowest with
Protamex® (6.85%), with an intermediate final DH of 9.35% with Neutrase®.
Alcalase® showed a higher efficiency than the two others for the hydrolysis of the cod muscle and was choose for the optimisation of the process.
54
Influence of the experimental conditions on the hydrolysis process in fish hydrolysates.
3.2. OPTIMIZATION OF PROCESSING CONDITIONS USING ALCALASE®
In a first time, the hydrolysis factors such as  temperature, time, and the amount of enzyme were changed to obtained different fractions with the same raw material, cod muscle. The process is reproducible and the inactivation of endogenous enzymes before the hydrolysis permit to follow each parameter. The hydrolysis degree was calculated with the two methods.
temperature, time, and the amount of enzyme were changed to obtained different fractions with the same raw material, cod muscle. The process is reproducible and the inactivation of endogenous enzymes before the hydrolysis permit to follow each parameter. The hydrolysis degree was calculated with the two methods.
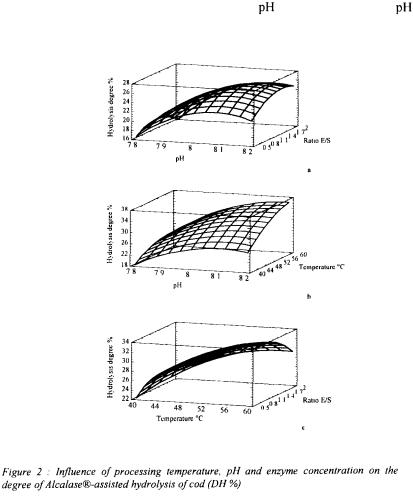
These following figures show the experimental response, in three dimensions under
the form of surface plots, of the combined effects of |
and ratio E/S (a), of |
and |
temperature (b), and of ratio E/S and temperature (c).
Each graphic is the representation of the evolution of the hydrolysis degree function of the experimental factors. Statistical analysis indicated that within each term all three hydrolysis factors had influence on DH. In fact, Adler-Nissen (1986), investigating the hydrolysis of soy protein by bacterial endoproteases, pointed out the  temperature
temperature
55
Rozenn Ravallec-Ple, Laura Gilmartin, Alain Van Wormhoudt and Yves Le Gal
and enzyme-substrate ratio markedly influenced the peptide bond cleavage in the protein substrate.
The highest degree of hydrolysis is obtained with high ratio and  even if important temperature reduce the extent of the process with the time of the hydrolysis. The largest surface is obtained with the combined
even if important temperature reduce the extent of the process with the time of the hydrolysis. The largest surface is obtained with the combined 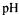 and the temperature, what could indicate that these two parameters are more important than the amount of enzyme.
and the temperature, what could indicate that these two parameters are more important than the amount of enzyme.
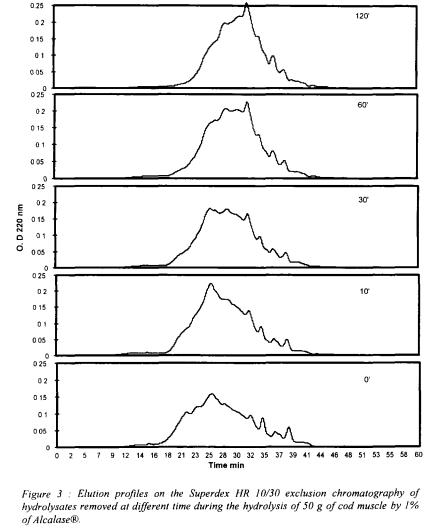
3.2. CHROMATOGRAPHIC PROFILES
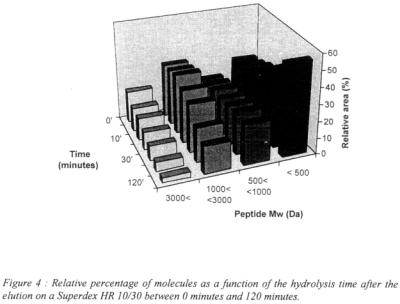
The evolution of the peptidic size profile as a function of the time was represented in the figure 3.
56
Influence of the experimental conditions on the hydrolysis process in fish hydrolysates.
With the increasing time and the extend of hydrolysis, the size distribution is varying and shows an increase of the low molecular weight peptides what is in correlation with the protein degradation and solubilisation during the process.
Figure 4 gives the relative area percentage (corresponding to the area under the curve of the different range of molecular weight) as a function of the time.
The most significant area increase is obtained with molecules of molecular weight under 500 daltons, corresponding to the decrease of molecules with a weight superior at 1000 daltons. Between 500 and 1000 daltons, the amount of peptides seems to stay constant during the process.
4. Conclusion
The degradation depends of the characteristics of the enzyme. Alcalase® is a bacterial endopeptidase with low specificity and is relatively effective for hydrolysis of fish proteins (Mohr, 1978). The combined effect of each pair of variable indicate that in the hydrolysis of cod muscle proteins an increase in DH is achieved by increases in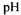 and temperature, more than with increases enzyme-substrate ratio, up to certain levels, beyond which DH slightly decreases. Such decrease in the percentage hydrolysis over the higher temperature values is explained by the increasing denaturation of the protease, reducing its biological activity (Diniz and Martin, 1997). The elution profiles on Superdex HR 10/30 gave further indications on the evolution of the hydrolysate composition during the process.
and temperature, more than with increases enzyme-substrate ratio, up to certain levels, beyond which DH slightly decreases. Such decrease in the percentage hydrolysis over the higher temperature values is explained by the increasing denaturation of the protease, reducing its biological activity (Diniz and Martin, 1997). The elution profiles on Superdex HR 10/30 gave further indications on the evolution of the hydrolysate composition during the process.
57
Rozenn Ravallec-Ple, Laura Gilmartin, Alain Van Wormhoudt and Yves Le Gal
Further in vitro and in vivo tests will complete these preliminary results. The combination of these tools with biological tests will give more information about the peptides present in the final product and could be of important interest for the determination of the best conditions to obtain high quality fish by-products.
References
Adler-Nissen, J. (1979) Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulphonic acid, J. Agric. FoodChem. 27(6), 1256-62.
Adler-Nissen, J. (1982) Limited enzymatic degradation of proteins: a new approach in the industrial application of hydrolases, J. Chem Tech. Biotechnol. 32, 138-156.
Adler-Nissen, J. (1986) Enzymatic hydrolysis of food proteins, Elsevier Applied Science Publishers, Barking, UK, 9-24, 57-109, 110-131.
Anonymous (1988) Alcalase® Food Grade, B 318b-GB 2000, Bagsvaerd, Denmark: Novo Industry A/S. Anonymous (1991) Enzymatic Modification of Proteins using Novo Nordisk Proteases, B 163g-GB 2500,
Bagsvaerd, Denmark: Novo Industri A/S.
Boyce, C. O. L. (1986) Nova’s Handbook of Practical Biotechnology, Bagsvaerd, Denmark: Novo Industri
A/S, 125.
Cancre, I., Ravallec, R., Van Wormhoudt, A., Stenberg, E., Gildberg, A. and Le Gal, Y. (1999) Secretagogues and growth factors in fish and crustacean protein hydrolysates, Mar Biotechnol 1 , 489494.
Chakraborty, P. K. and Madhavan, P. (1977) A pilot scale set up for the manufacture of fish hydrolysate.
Fish. Technol. 14(2), 159-158.
Diniz, F. M. and Martin, A. M. (1997) Optimisation of nitrogen recovery in the enzymatic hydrolysis of dogfish (Squalus acanthias) proteinComposition of the hydrolysates, Int. J. Food Sci. Nutrit. 48, 191-
200.
Fouchereau-Peron, M., Duvail, L., Michel, C., Gildberg, A., Batista, I. and Le Gal, Y. (1999) Isolation of an acid fraction from a fish protein hydrolysate with a calcitonin-gene-related-peptide-like biological activity, Biotechnol. Appl. Biochem. 29, 87-92.
Guérard, F., Dufossé, L., De La Broise, D., Binet, A. (2000) Enzymatic hydrolysis of proteins from yellowfin tuna (Thunnus albacares) wastes using alcalasc. Journal of Molecular Catalysis B :
Enzymatic, In Press.
Gildberg, A., Johansen, A. and Bogwald, J. (1995) Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida., Aquaculture 138 , 23-24.
Lynch, J. M., Barbano, D. M. and Fleming, J. R. (1998) Indirect and direct determination of the casein content of milk by Kjeldahl nitrogen analysis: collaborative study, J. AOAC Int. 81(4), 763-774.
Mohr, V. (1978) Fish protein concentrate production by enzymatic hydrolysis, in Biochemical aspects of new protein foods , J. Adler-Nissen, B. D. Eggum, L. Munck and H. S. Olsen eds, Proc. 11th FEBS
Meeting, Pergamon Press, Oxford, 44, 53-62.
Piot, J. M., Zhao, Q., Guillochon, D., Ricart, G. and Thomas, D. (1992) Isolation and characterisation of two opioïd peptides from a bovine haemoglobin peptidic hydrolysate, Biochem. Biophys. Res. Commun 189, 101-110.
Quaglia, G. B. and Orban, E.(1987a) Enzymatic solubilisation of proteins of sardines (Sardina pilchardus) by commercial proteases, J. Sci. Food Agric. 38, 263-269.
Quaglia, G. B. and Orban, E.(1987b) Influence of the degree of hydrolysis on the solubility of the protein hydrolysates from sardines (Sardina pilchardus), J. Sci. Food Agric. 38, 271-276.
Ravallec-Plé, R., Gilmartin, L., Van Wormhoudt, A. and Le Gal, Y. (2000), Influence of the hydrolysis process on the biological activities of the protein hydrolysates from cod muscle (Gadus morhua). J. Sci. Food and Agr. (submitted).
Shahidi, F., Naczk, M., Pegg, R.B. and Synowiecki, J. (1991) Chemical composition and nutritional value of processing discards of cod (Gadus morhua), Food Chem 42 , 145-151.
Venugopal, V. (1994) Production of fish protein hydrolysates by microorganisms. In Fisheries Processing : Biotechnological applications, A. M. Martin ed., Chapman & Hall Press, London, 223-243.
58
