
Engineering and Manufacturing for Biotechnology - Marcel Hofman & Philippe Thonart
.pdf10.3.4. The EPO option ............................................................................ |
481 |
|||
10.3.5. Country selection.......................................................................... |
482 |
|||
10.3.6. Annuity fees................................................................................. |
482 |
|||
11 . Patent Position in Action................................................................................ |
482 |
|||
11.1. Patent claims versus products.................................................................. |
482 |
|||
11.2. Litigation versus license .......................................................................... |
483 |
|||
11.2.1. Litigation ...................................................................................... |
483 |
|||
11.2.2. |
Licensing...................................................................................... |
483 |
||
11.2.3. More |
timing.................................................................................. |
484 |
||
11.3. Survival claim |
reading............................................................................. |
484 |
||
11.3.1. Look only to the words of the claims ........................................... |
484 |
|||
11.3.2. Element-by-element comparison.................................................. |
484 |
|||
11.3.3. Numerical claim limitations.......................................................... |
484 |
|||
11.3.4. |
Ignore |
predicate phrases............................................................... |
484 |
|
11.3.5. |
Terms |
of art.................................................................................. |
485 |
|
11.3.6. |
Definitions.................................................................................... |
485 |
||
11.3.7. |
File |
wrapper estopped................................................................... |
485 |
|
11.3.8. Things that won’t help................................................................... |
485 |
|||
12. Digging for patent dirt.................................................................................... |
485 |
|||
12.1. File histories |
............................................................................................ |
|
485 |
|
12. 2. Computer searching................................................................................ |
486 |
|||
Conclusion............................................................................................................ |
|
|
|
486 |
INDEX........................................................................................................ |
|
|
|
487 |
18
PART I
UPSTREAM PROCESSES AND FERMENTATION
PRETREATMENT PROCESSES OF MOLASSES FOR THE UTILIZATION IN FERMENTATION PROCESSES
GÜZIDE ÇALIK, MELIHA BERK, FATMA GÜL BOYACI, PINAR ÇALIK, SERPIL TAKAÇ, TUNÇER H. ÖZDAMAR
Ankara University Biotechnology Research Center, Industrial
Biotechnology Department,  06100, Ankara, TURKEY
06100, Ankara, TURKEY
Abstract
The composition of beet molasses was modified by 14 pretreatment processes which were the combination of physical, physicochemical, and chemical processes, i.e. acid hydrolysis of sucrose, precipitation of metal ions, removal of organic acids by ion exchange resins, and entrapment of metal ions in solution. Acid hydrolysis was favourable for glutamic acid fermentation, while the use of diluted and centrifuged molasses was advantageous for alkaline protease fermentation.
1. Introduction
Molasses, which are the by-products of the sugar-beet or sugarcane extraction processes are among the most important raw materials of the fermentation industry; especially for the production of baker's yeast, citric acid, feed yeasts, acetone/butanol, organic acids, amino acids, antibiotics, and enzymes. The suitability of molasses for industrial fermentations cannot be evaluated according to their origin and chemical composition as different criteria determine the productivity and quality for their use in different processes. In processes in which they are the sole carbon source, the molasses should be pretreated and the inhibitors should be removed. For yeast and methanol production molasses are often simply neutralised with calcium carbonate. For many other processes they are only boiled in an acidic or alkaline medium and after setting out separated from the precipitate. For citric acid production the molasses are boiled with potassium ferrocyanide and generally fermented together with the precipitate (Palacios, 1966, Kundu et al, 1984, Cejka, 1985, Sharma et al., 1991).
Although beet or cane molasses has been used in the fermentation media for the production of glutamic acid with Brevibacterium or Corynebacterium strains in literature the aim were generally to investigate the effect of other operational parameters
21
M. Hofman and P. Thonart (eds.), Engineering and Manufacturing for Biotechnology, 21–28.
© 2001 Kluwer Academic Publishers. Printed in the Netherlands.

Güzide Çalik, et al
such as the use of surface active agents to increase the membrane permeability in fedbatch operation (Yu-cheng, 1973), shift in operation temperature with temperature sensitive mutants (Momose and Takagi, 1978), estimation of cell growth with on-line
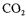 measurement (Park et al., 1983a,b, 1984, Wu et al., 1989), continuous production with immobilised biocatalyst (Kim and Ryu 1982). The pretreatment processes, if applied any, has not been described in the above-mentioned literature.
measurement (Park et al., 1983a,b, 1984, Wu et al., 1989), continuous production with immobilised biocatalyst (Kim and Ryu 1982). The pretreatment processes, if applied any, has not been described in the above-mentioned literature.
The effects of various defined and semi-defined media involving simple carbon sources such as glucose and/or fructose, organic acids, and amino acids, or complex carbon sources such as casein, corn steep liquor, and starch (Çalik et al., 1998, Çalik et al., 2000), in the production of serine alkaline proteases that are the most important group of industrial enzymes using Bacillus strains have been investigated, however molasses has not been used for the protease production processes in the literature.
This work investigates the effects of several pretreatment processes (PP), which were the combination of physical, physicochemical, and chemical processes, systematically on the composition of molasses and finally on the efficiency of their use on two bioprocesses, namely, glutamic acid and serine alkaline protease production.
2. Materials and methods
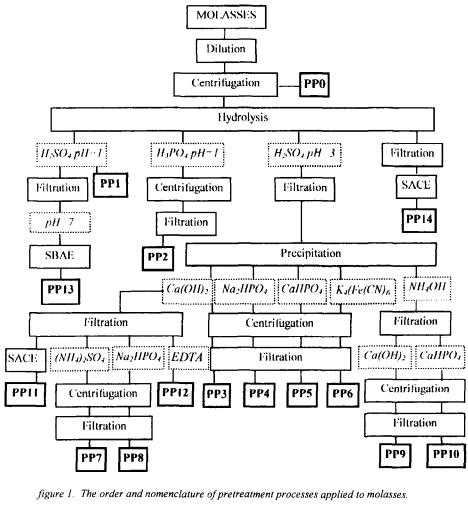
2.1. PRETREATMENT PROCESSES (PP)
Beet molasses, the composition and properties of which was given in Table 1 was modified with 14 pretreatment processes that have been designed as the combination of the following unit processes and abbreviated as PP1 - PP14 as given in Figure 1 (Berk, 1995). Not pretreated, that is, only diluted and centrifuged molasses was abbreviated as
PP0. The details of the physical, physicochemical, and chemical pretreatment shown in the Figure are as follows: Dilution: 100 g molasses was diluted with water to obtain solution. Centrifugation: Diluted or insoluble impurity containing molasses was centrifuged for 20 min at 6000 g and  conditions. Hydrolysis: Sucrose
conditions. Hydrolysis: Sucrose
hydrolysis of molasses was accomplished either with a liquid acid, i.e. |
and |
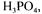 or a solid acid, i.e. Amberlite IR-120P. Liquid acid hydrolysis was made at temperature,
or a solid acid, i.e. Amberlite IR-120P. Liquid acid hydrolysis was made at temperature, 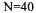 rpm agitation rate, and
rpm agitation rate, and  h conditions with 6M
h conditions with 6M
acid. In some of the pretreatments further hydrolysis with liquid at |
was made in |
22

Pretreatment processes of molasses for the utilization in fermentation processes
order to hydrolyse sucrose completely. Solid hydrolysis reaction was established in packed column including strongly acidic cation exchange resin, Amberlite IR-120P, at 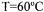 temperature and
temperature and 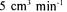 flow rate conditions by using molasses solution containing
flow rate conditions by using molasses solution containing 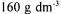 sucrose. Precipitation: Metal ions in molasses were precipitated
sucrose. Precipitation: Metal ions in molasses were precipitated
with |
|
|
|
and |
|
solutions, |
precipitation was carried out either at |
or |
and |
h, |
rpm, |
||
and |
temperature conditions with |
1.5 M base. |
|
precipitation was similar to |
||
|
In the precipitation with |
and |
|
|
|
and |
|
were added separately to molasses solution to adjust pH to 3 and |
|||||
8, respectively. The reaction was carried out for 1 h at |
temperature. To separate |
|||||
ions |
from the medium |
was added. |
precipitation was |
|||
performed |
with |
solution. |
EDTA Entrapment: |
5 % |
(w/v), |
|
 EDTA was used in order to entrap metal ions from the solution. SACE Pretreatment: In one of the pretreatment processes metal ions were removed with a strongly acidic cation exchange (SACE) resin, Amberlite IR-120P. For this purpose, molasses solution was fed to the ion exchange column at
EDTA was used in order to entrap metal ions from the solution. SACE Pretreatment: In one of the pretreatment processes metal ions were removed with a strongly acidic cation exchange (SACE) resin, Amberlite IR-120P. For this purpose, molasses solution was fed to the ion exchange column at  flow rate. SBAE Pretreatment: Organic acids were removed with a strongly basic anion exchange
flow rate. SBAE Pretreatment: Organic acids were removed with a strongly basic anion exchange
(SBAE) resin, Amberlite IRA-400. After the pH of  molasses solution had adjusted to 7, it was fed to the ion exchange column at
molasses solution had adjusted to 7, it was fed to the ion exchange column at 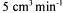 flow rate. Filtration: The solution was filtered with Whatman No:l filter paper after the hydrolysis and precipitation steps for the separation of the solid impurities.
flow rate. Filtration: The solution was filtered with Whatman No:l filter paper after the hydrolysis and precipitation steps for the separation of the solid impurities.
2.2. BIOPROCESSES
2.2.1. Glutamic acid fermentation
Corynebacterium glutamicum (NRRL B-2784) was grown, inoculated and cultivated as described elsewhere (Berk, 1995). 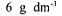 urea was added to molasses solution involving
urea was added to molasses solution involving 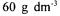 reduced sugar. pH was adjusted with 25 %
reduced sugar. pH was adjusted with 25 % 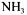 to 7.3. Batch experiments were conducted in agitation and heating rate controlled orbital shakers,
to 7.3. Batch experiments were conducted in agitation and heating rate controlled orbital shakers,
using microbial air filtered |
flasks having |
working volume capacities at |
temperature and |
rpm agitation |
rate conditions. |
penicillin G was added to the bioconversion medium at  h cultivation time.
h cultivation time.
2.2.2. Alkaline protease fermentation
Bacillus licheniformis (DSM 1969) was grown, inoculated and cultivated as described elsewhere (Çalik et al., 1998). The reference medium for the investigation of pretreatment processes in alkaline protease fermentation was 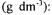 glucose, 6.0;
glucose, 6.0;
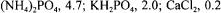 (Çalik et al., 1998). Glucose was replaced by pretreated molasses solution PP0, PP1, and PP13 in order to investigate the effects of the pretreatment. Batch experiments were conducted in agitation and heating rate controlled orbital shakers, using microbial air filtered
(Çalik et al., 1998). Glucose was replaced by pretreated molasses solution PP0, PP1, and PP13 in order to investigate the effects of the pretreatment. Batch experiments were conducted in agitation and heating rate controlled orbital shakers, using microbial air filtered  flasks having
flasks having 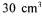 working volume capacities at
working volume capacities at 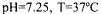 temperature and
temperature and  rpm agitation rate conditions.
rpm agitation rate conditions.
23
Güzide Çalik, et al
3.Results and discussions
3.1.EFFECT OF PP ON METAL ION CONCENTRATIONS
The beet molasses solution contains compounds used as substrates in bioprocesses such as sucrose, invert sugar, amino acids, organic acids, inorganic compounds and vitamins (Schneider,1979; Cejka,1985). However, some compounds can inhibit growth Table 2. The efficiency of metal ion removal with the pretreatment processes for molasses of the microorganism and product formation. Moreover, the stability of the product can be decreased by the compounds involved in molasses. Consequently, the molasses must be
24
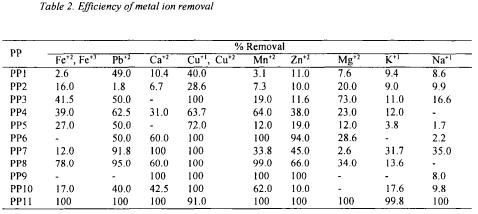
Pretreatment processes of molasses for the utilization in fermentation processes
modified by some pretreatment processes before using as a substrate in bioprocesses.
One of the objectives of this work was to remove the metal ions, 
 and
and 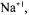 by pretreatment processes and to determine the pretreatment process effects in glutamic acid and alkaline protease fermentations. The order of different pretreatment processes designed in this work, which were the combination of dilution, centrifugation, acid hydrolysis of sucrose, precipitation of metal ions, removal of organic acid, entrapment of metal ions and filtration are given in detail in Figure 1. After the employment of the pretreatment processes from PP1 to PP11, the efficiency of metal ion removal can be seen in Table 2. The metal ions were removed completely by the pretreatment processes PP12 and PP14.
by pretreatment processes and to determine the pretreatment process effects in glutamic acid and alkaline protease fermentations. The order of different pretreatment processes designed in this work, which were the combination of dilution, centrifugation, acid hydrolysis of sucrose, precipitation of metal ions, removal of organic acid, entrapment of metal ions and filtration are given in detail in Figure 1. After the employment of the pretreatment processes from PP1 to PP11, the efficiency of metal ion removal can be seen in Table 2. The metal ions were removed completely by the pretreatment processes PP12 and PP14.
3.2. EFFECT OF PP ON GLUTAMIC ACID FERMENTATION
The glutamic acid fermentation was carried out by PP0, PP1, PP2, PP3, PP7, PP11 and PP14 molasses for 37 h. The effect of the pretreatment processes on the relative concentrations of Corynebacterium glutamicum and glutamic acid are shown in Figures
2 and 3, respectively. As it is clear in Figure 2, pretreatment processes inhibit cell growth to some extent, where PP14 has the most detrimental effect, because all the metal ions are removed totally. When glutamic acid concentration is considered the best results were achieved with PP1, PP13 and PP2. The comparison of results of fermentations carried out with untreated and pretreated molasses showed that the pretreated molasses increased the glutamic acid yield up to 70 %. The highest glutamic acid concentration was obtained with PP1. In PP1,  and
and 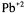 ions were precipitated together with
ions were precipitated together with 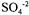 ions as their sulphates; however, in
ions as their sulphates; however, in 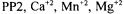 and
and  ions precipitate as their phosphates. From the results given in Figure 2 one can conclude that phosphate ion is still a stronger inhibitor than sulphate ion for the microorganism in glutamic acid fermentation. Therefore, its concentration in the media should be very low. Organic acids were removed by strongly basic anion exchange resin with
ions precipitate as their phosphates. From the results given in Figure 2 one can conclude that phosphate ion is still a stronger inhibitor than sulphate ion for the microorganism in glutamic acid fermentation. Therefore, its concentration in the media should be very low. Organic acids were removed by strongly basic anion exchange resin with
PP13. PP13 was almost as effective as PP1, hence the concentration of organic acids does not produce a negative effect in glutamic acid fermentation.
25

3.3. EFFECT OF PP ON SERINE ALKALINE PROTEASE FERMENTATION
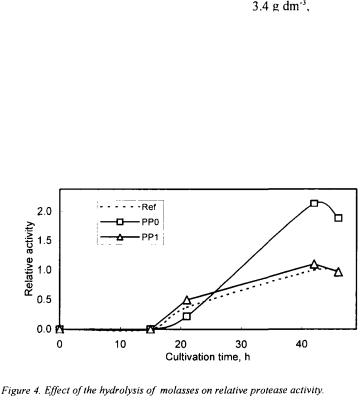
The effect of pretreatment processes on alkaline protease fermentation was investigated with PP0, PP1, and PP13 molasses. Alkaline protease productions were carried out for
26
Pretreatment processes of molasses for the utilization in fermentation processes
 h. For this purpose, readily accessible carbon source glucose in the fermentation media was replaced with PP0, PP1, and PP13 molasses corresponding to different sucrose or reduced sugar concentrations between
h. For this purpose, readily accessible carbon source glucose in the fermentation media was replaced with PP0, PP1, and PP13 molasses corresponding to different sucrose or reduced sugar concentrations between  The variations of cell concentration and enzyme activity were measured throughout the bioprocess. The activity and cell concentrations obtained with PP1 and PP13 molasses did not differ significantly. The results showed that when sucrose/reduced sugar concentration of the PP0 and PP1 molasses increased, the cell concentration first increased and then decreased, giving a maximum at 45 and
The variations of cell concentration and enzyme activity were measured throughout the bioprocess. The activity and cell concentrations obtained with PP1 and PP13 molasses did not differ significantly. The results showed that when sucrose/reduced sugar concentration of the PP0 and PP1 molasses increased, the cell concentration first increased and then decreased, giving a maximum at 45 and 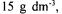 respectively. With PP0 and PP1
respectively. With PP0 and PP1
molasses, maximum cell concentrations were 2.5 and |
, |
while for the reference |
medium involving  glucose as the carbon source this value was
glucose as the carbon source this value was  Reduced sugar concentration affected the enzyme activity in the same manner and
Reduced sugar concentration affected the enzyme activity in the same manner and 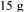
 gave maximum relative activity with PP1 when compared with the reference solution, as 1.1 and it was obtained at
gave maximum relative activity with PP1 when compared with the reference solution, as 1.1 and it was obtained at  h. However, only diluted and centrifuged molasses PP0 was more effective in the protease production with the relative activity value 2.1 obtained with
h. However, only diluted and centrifuged molasses PP0 was more effective in the protease production with the relative activity value 2.1 obtained with  sucrose containing molasses (Figure 4). These results show that besides the positive effects of amino acids, organic acids, inorganic compounds and vitamins involved in molasses the use of sucrose instead of a readily accessible carbon source, e.g. glucose and fructose, may cause the microorganism to function the bioreaction network under stress that it increases the protease production.
sucrose containing molasses (Figure 4). These results show that besides the positive effects of amino acids, organic acids, inorganic compounds and vitamins involved in molasses the use of sucrose instead of a readily accessible carbon source, e.g. glucose and fructose, may cause the microorganism to function the bioreaction network under stress that it increases the protease production.
4. Conclusions
14 pretreatment processes were developed for the utilisation of beet molasses in fermentation process, i.e., glutamic acid and serine alkaline protease. The best results were obtained with PP1 for glutamic acid production, however, PP0 molasses was more beneficial for the protease enzyme production. When essential components are removed from the media, the cell growth is strongly inhibited. This consequently causes a decrease in the product formation. However, the presence of some ions may induce the stability of a product, e.g. an enzyme as well. Therefore, the design of the pretreatment
27
Güzide Çalik, et al
process to be applied to a complex carbon and energy source should be made considering the needs of the cell and its metabolism. The investigation of different pretreatment processes to protease enzyme production as well as other bioprocesses are being continued.
Acknowledgements
The financial support of SPO (Turkey) Grant No. 89K120390 and 95K120290 are gratefully acknowledged.
References
Berk, M. (1995) Adaptation of molasses to the bioprocess for L-glutamic acid, M.S. Thesis (Turkish), Ankara University, Ankara.
Cejka, A. (1985), Preparation of media, in: H.-J.Rehm and G. Reed (eds.), Biotechnology, VCH,
Weinheim, 2, pp. 629-639.
Cohen, A. (1984) The Pico.Tag system: A new method to analyse primary and secondary amino acids with one picomole sensitivity, Bio.Tech., September-October, 273-279.
Çalik, P., Çalik, G. and Özdamar, T.H. (1998) Oxygen transfer effects in serine alkaline protease fermentation by Bacillus licheniformis. Use of citric acid as the carbon source, Enzyme Microb. Technol. 23,451-461.
Çalik, P., Çalik, G., and Özdamar, T.H. (in press) Bioprocess development for serine alkaline protease production, Reviews in Chemical Engineering.
Kim, H.. and Ryu, D.D.Y. (1982) Continuous glutamate production using an immobilised whole-cell system, Biotechnol. Bioeng., 24, 2167-2174.
Kundu, ST., Panda, S.K., Majumdar, B., and Guha (1984) Pretreatment of Indian cane molasses for increased production of citric acid, Biotechnol. Bioeng. 26, 1114-1121.
Momose, H. and Takagi, T. (1978) Glutamic acid production in biotin-rich media by temperature sensitive mutants of Brevibacterium lactofermentum, a novel fermentation process, Agric. Biol. Chem. 42, 19111917.
Palacios, D.R. (1966) Citric acid from ferrocyanide-treated blackstrap molasses, Biotechnol. Bioeng., 11, 103-104.
Park, S.H., Hong, K.T., Lee, J.C., and Bea, J.C. (1983a) On-line estimation of cell growth for glutamic acid fermentation system, Eur. J. Appl, Microb. Biotechnol., 17, 168-172. Korean J. Food Sci. Technol., 15, Park, S.H., Hong, K.T., You, S.J., and Lee, J.C. (1983b) Automation of glutamic acid fermentation, Korean
J. Food Sci. Technol., 15, 202-204.
Hong, K.T., Park, S.H., Lee, J.C., Choi, C.Y. and Bae, J.C. (1984) Control of sugar feeding for glutamic acid fermentation, J. Ferment Technol.., 62, 49-54.
Schneider, F. (1979) Sugar analysis official and tentative methods recommended by the international commission for uniform methods of sugar analysis (ICUMSA), England.
Sharma, C.B. and Garg, K. (1991) Repeated batch production of citric acid from sugarcane molasses using recycled solid-state surface culture of Aspergillus niger, Biotechnol. Letters 13, 913-916.
Wu, W., Tsao, J., and Fan, C. (1989) On-line estimation of cell mass in glutamic acid production, Journal of
Fermentation and Bioengineering 3, 220-221.
Yu-cheng, C. (1973) MSG production by the fermentation of cane molasses, Taiwan Sugar 5, 180-184.
28
