
- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
2. What was said in the text about:
the origin of the word “stereochemistry”;
the foundation of organic stereochemistry;
enantiomers;
chirality;
stereogenic center.
3. Put five special questions to the text and mark parts of the text which give the answers for them.
4. Find the key paragraphs of the text.
5. Render the text according to the scheme (Appendix 3).
Section B
Read the text below and decide which answer A, B, C or D best fits each gap.
PROPERTIES OF CHIRAL MOLECULES: OPTICAL ACTIVITY
The experimental facts that led van’t Hoff and Le Bel to propose that molecules having the same constitution could differ in the arrangement of their atoms in space concerned the physical property of optical activity. Optical activity is the ability of a chiral substance to rotate the plane of plane-polarized light and is measured using an instrument called a polarimeter.
The light used to measure optical activity has two properties: it consists of a single wavelength and it is plane-polarized. The wavelength used most often is 589 nm (called the D line), which corresponds to the yellow light produced by a sodium lamp.
Except for giving off light of a single wavelength, a sodium lamp is like any other lamp in that its light is 1)___________, meaning that the plane of its electric field vector can have any orientation along the line of travel. A beam of this light is transformed to 2)___________ light by passing it through a 3) _________, which removes all the waves except those that have their electric field vector in the same plane. This plane- polarized light now passes through the 4)___________ containing the substance to be examined, either in the liquid phase or as a solution in a suitable solvent (usually water, ethanol, or chloroform). The sample is 5)“______________” if it rotates the plane of polarized light. The direction and magnitude of rotation are measured using a second polarizing filter (the “analyzer”) and cited as, the observed rotation.
To be optically active, the sample must contain a chiral substance and one enantiomermust be present in excess of the other. Asubstance that does not rotate the plane of polarized light is said to be optically inactive. All achiral substances are optically inactive.
|
A polarized |
B unpolarized |
C oriented |
D filtered |
|
A plane-unpolarized |
B plane-oriented |
C plane-polarized |
D plane |
|
A polarized light |
B polarizer |
C polarizing plane |
D polarizing filter |
|
A sample test |
B sample tube |
C optical substance |
D tube with solution |
|
A optically inactive |
B polarized |
C unpolarized |
D optically active |
Check your answers to the ex. 1 with the picture below.
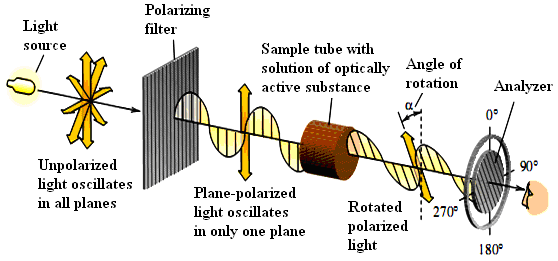
Explain the principles of work of the polarimeter.
The text belowshows how the Cahn–Ingold–Prelog system, called the sequence rules, is used to specify the absolute configuration at the stereogenic center in (+)-2-butanol. Put the steps of the system into correct order.
Absolute configuration according to the Cahn–Ingold–Prelog Notational System
gives that the absolute configuration of (+)-2-butanol is
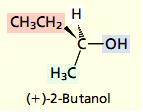
1. Orient the molecule so that the lowest ranked substituent points away from you.
2. If the order of decreasing precedence of the three highest ranked substituents appears in a clockwise sense, the absolute configuration is R (Latin rectus, “right,” “correct”). If the order of decreasing precedence is anticlockwise, the absolute configuration is S (Latin sinister, “left”).
3. Identify the substituents at the stereogenic center, and rank them in order of decreasing precedence according to the system described in Section 5.4. Precedence is determined by atomic number, working outward from the point of attachment at the stereogenic center.
4. Draw the three highest ranked substituents as they appear to you when the molecule is oriented so that the lowest ranked group points away from you.
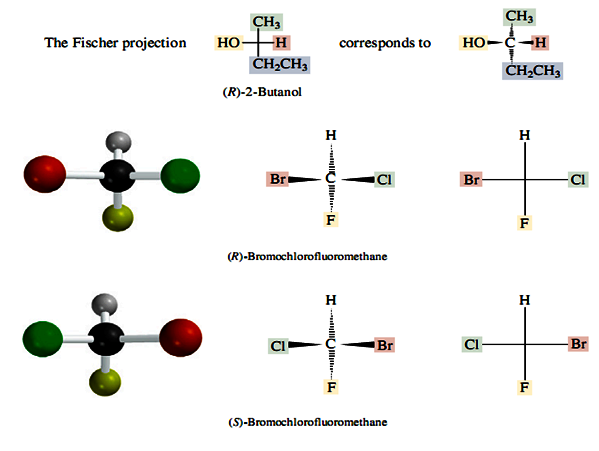
Looking at the figure below explain the principles of the Fischer projection.
Speaking
Using conversational gambits given in the Appendix 2 construct a dialogue “Why do I like (dislike) stereochemistry”.
Match the following terms with their definition and give any additional information about them:
|
1 Stereoisomers |
a) Stereoisomers that are not enantiomers | |
|
2 Enantiomers |
b)One that rotate plane-polarized light are optically active (chiral). Compounds designated "l" rotate the plane of the polarized light counterclockwise. Compounds designated "d" rotate the plane of the polarized light clockwise | |
|
3 Diastereomers |
c)An sp3carbon with four different substituents, often referred to as a chiral carbon. Stereoisomers are formed by changing the orientation of the substituents of the chiral carbon. | |
|
4 Asymmetric carbon |
d)Isomers that have identical connectivity but different spatial configurations: two types are enantiomers and diastereomers. | |
|
5 Optically active compounds |
e)If you can draw an internal mirror plane through a molecule (drawing the plane down the center of an atom is allowed), the molecule is not optically active and is called achiral. | |
|
6 General rule of thumb |
f)Stereoisomers that are nonsuperimposable mirror images of each other | |
