
- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
Asymmetric Synthesis
Organic chemists often wish to synthesize a chiral compound in the form of a single enantiomer or diastereomer, rather than as a mixture of stereoisomers. There are two basic ways in which this can be done. The first way, which is more common, is to begin with a single stereoisomer, and to use a synthesis that does not affect the chiral center (or centers), as in the glyceraldehyde-glyceric acid. The optically active starting compound can be obtained by a previous synthesis, or by resolution of a racemic mixture, but it is often more convenient to obtain it from nature, since many compounds, such as amino acids, sugars, and steroids are present in nature in the form of a single enantiomer or diastereomer. These compounds are regarded as a chiral pool; that is, readily available compounds that can be used as starting materials.
The other basic method is called asymmetric synthesis or stereoselective synthesis. As was mentioned before, optically active materials cannot be created from inactive starting materials and conditions; hence, true asymmetric synthesis is impossible, except in the manner previously noted. However, when a new stereogenic center is created, the two possible configurations need not be formed in equal amounts if anything is present that is not symmetric. We discuss asymmetric synthesis under four headings:
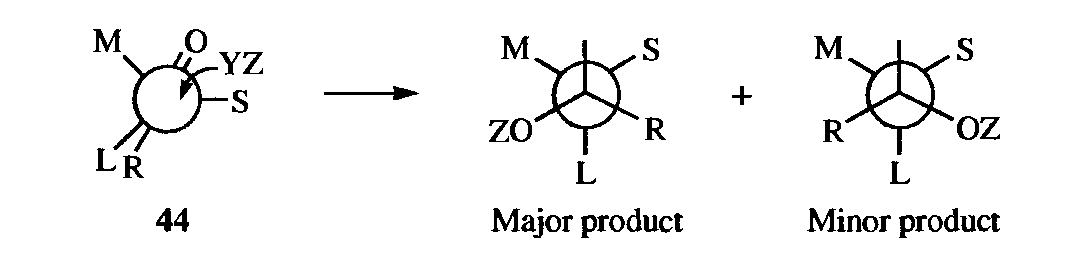
1. Active Substrate. If a new stereogenic center is created in a molecule that is already optically active, the two diastereomers are not (except fortuitously) formed in equal amounts. The reason is that the direction of attack by the reagent is determined by the groups already there. For certain additions to the carbon-oxygen double bond of ketones containing an asymmetric ɑ carbon, Cram's rule predicts which diastereomer will predominate (diastereoselectivity).
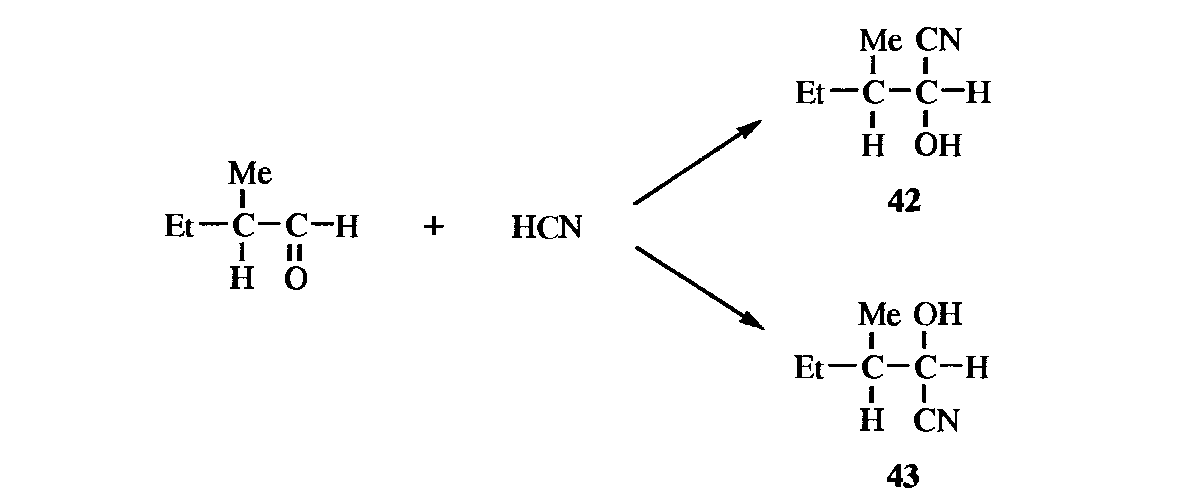
If the molecule is observed along its axis, it may be represented as in 44, where S, M, and L stand for small, medium, and large, respectively. The oxygen of the carbonyl orients itself between the small- and the medium-sized groups. The rule is that the incoming group preferentially attacks on the side of the plane containing the small group. By this rule, it can be predicted that 43 will be formed in larger amounts than 42.
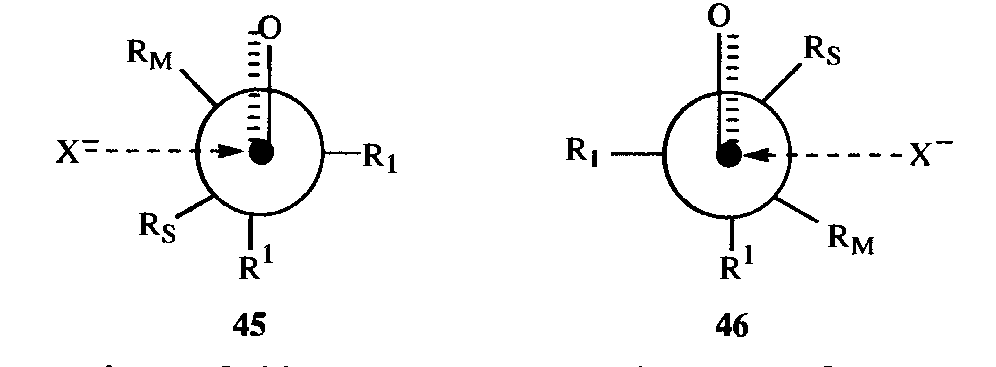
Another model can be used to predict diastereoselectivity, which assumes reactant-like transition states and that the separation of the incoming group and any electronegative substituent at the a carbon is greatest. Transition state models 45 and 46 are used to predict diastereoselectivity in what is known as the Felkin-Ahn model.
Many reactions of this type are known, in some of which the extent of favoritism approaches 100%. The farther away the reaction site is from the stereogenic center, the less influence the latter has and the more equal the amounts of diastereomers formed.
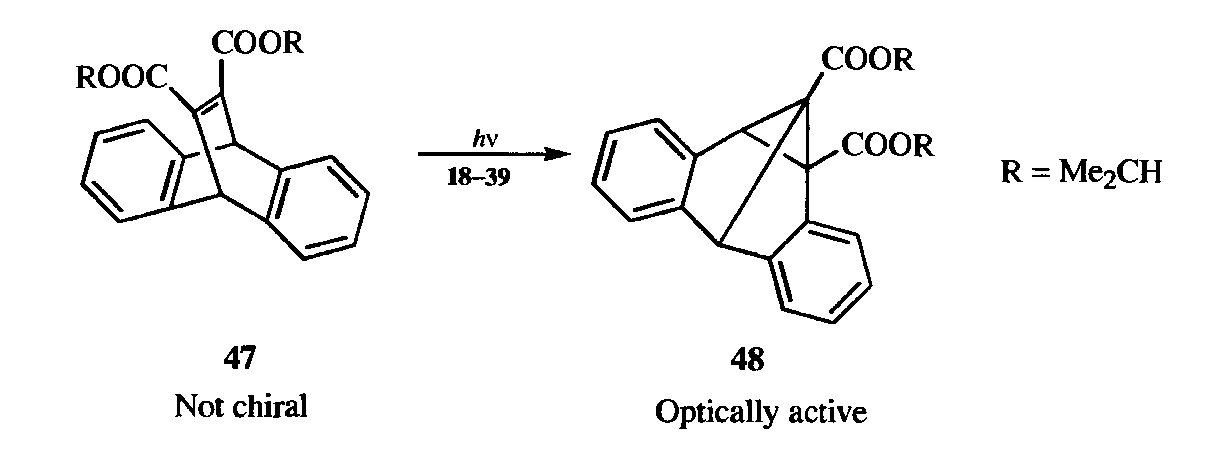
In a special case of this type of asymmetric synthesis, a compound (47) with achiral molecules, but whose crystals are chiral, was converted by UV light to a single enantiomer of a chiral product (48).
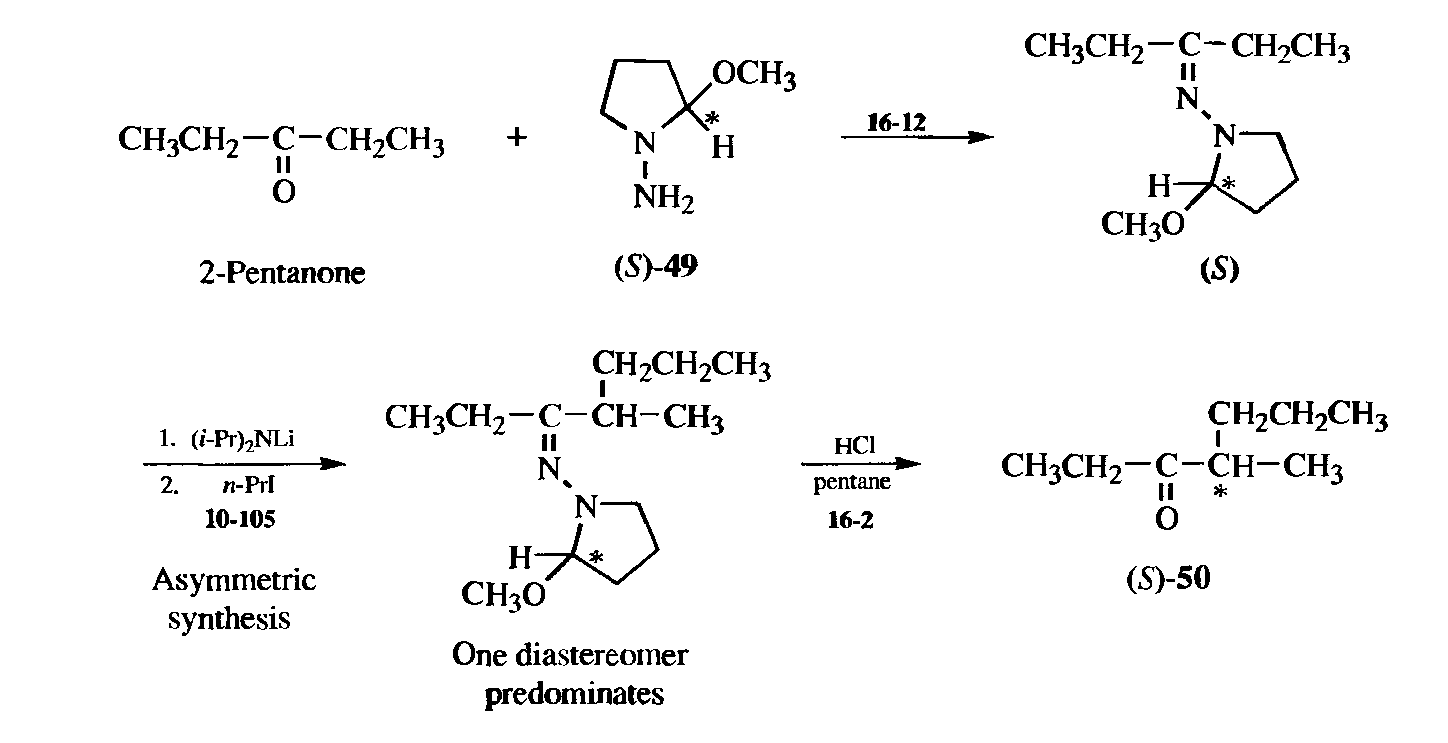
It is often possible to convert an achiral compound to a chiral compound by (1) addition of a chiral group; (2) running an asymmetric synthesis, and (3) cleavage of the original chiral group. An example is conversion of the achiral 2-pentanone to the chiral 4-methyl-3-heptanone (50). In this case, >99% of the product was the (S)enantiomer. Compound 49 is called a chiral auxiliary because it is used to induce asymmetry and then is removed.
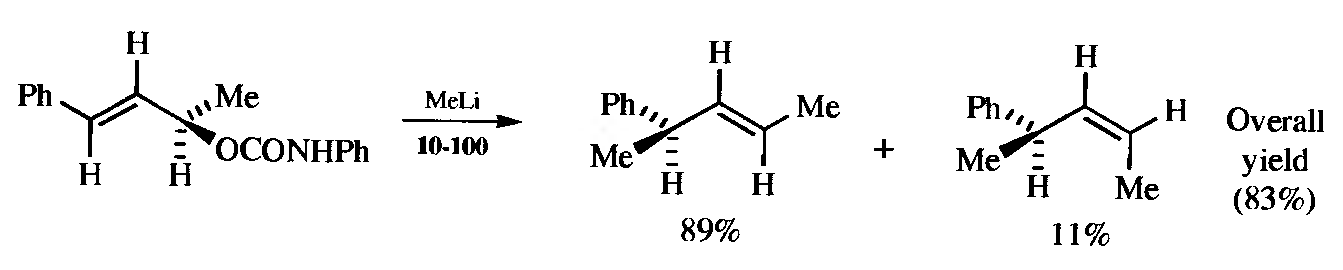
Active Reagent. A pair of enantiomers can be separated by an active reagent that reacts faster with one of them than it does with the other (this is also a method of resolution). If the absolute configuration of the reagent is known, the configuration of the enantiomers can often be determined by a knowledge of the mechanism and by seeing which diastereomer is preferentiallyformed.
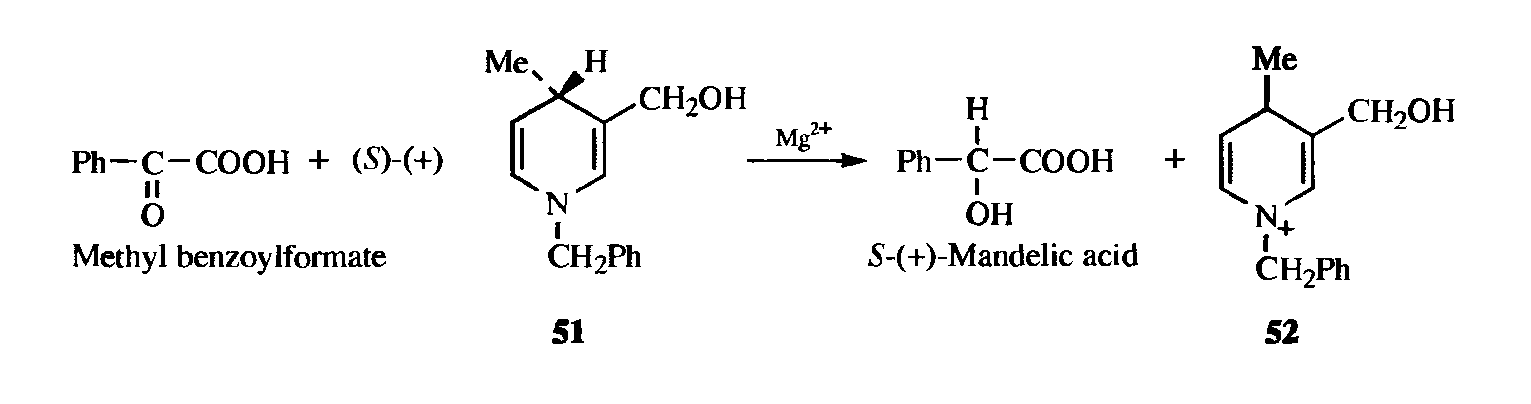
Creation of a new chiral center in an inactive molecule can also be accomplished with an active reagent, though it is rare for 100% selectivity to be observed. An example is the reduction of methyl benzoylformate with optically active N-benzyl-3-(hydroxymethyl)-4-methyl-1,4-dihydropyridine (51) to produce mandelic acid, which contained ~97.5% of the (S)-(+) isomer and 2.5% of the (R)-(-) isomer. Note that the other product, (52), is not chiral. Reactions like this, in which one reagent (in this case 51) gives up its chirality to another, are called selfimmolative. In this intramolecular example, chirality is transferred from one atom to another in the same molecule.
A reaction in which an inactive substrate is converted selectively to one of two enantiomers is called an enantioselective reaction, and the process is called asymmetric induction. These terms apply to reactions in this category and in categories 3 and 4.
When an optically active substrate reacts with an optically active reagent to form two new chiral centers, it is possible for both centers to be created in the desired sense. This type of process is called double asymmetric synthesis.
3. Active Catalyst or Solvent Many such examples are present in the literature, among them reduction of ketones and substituted alkenes to optically active (though not optically pure) secondary alcohols and substituted alkanes by treatment with hydrogen and a chiral homogeneous hydrogenation catalyst, the treatment of aldehydes or ketones with organometallic compounds in the presence of a chiral catalyst,and the conversion of alkenes to optically active epoxides by treatment with a hydroperoxide and a chiral catalyst. In some instances, notably in the homogeneous catalytic hydrogenation of alkenes, the ratio of enantiomers prepared in this way is as high as 98:2. Other examples of the use of a chiral catalyst or solvent are the conversion of chlorofumaric acid (in the form of its di-ion) to the (-)-threoisomer of the di-ion of chloromalic acid by treatment with H20 and the enzyme fumarase, and the preparation of optically active aldolsby the condensation of enolate anions with optically active substrates.
4. Reactions in the Presence of Circularly Polarized Light. If the light used to initiate a photochemical reaction of achiral reagents is circularly polarized, then, in theory, a chiral product richer in one enantiomer might be obtained. However, such experiments have not proved fruitful. In certain instances, the use of left- and right-circularly polarized light has given products with opposite rotations (showing that the principle is valid), but up to now the extent of favoritism has always been <1%.
