- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
Diastereomeric Relationships
Diastereomers include all stereoisomers that are not related as an object and its mirror image. These structures represent the four stereoisomers of 2,3,4-trihydroxybutanal. The configurations of C-2 and C-3 are indicated. Each stereogenic center is designated Ror S by application of the sequence rule. Each of the four structures is stereoisomeric with respect to any of the others. The 2R,3Rand 2S,3Sisomers are enantiomeric, as are the 2R,3Sand 2S,3Rpair. The 2R,3S isomer is diastereomeric with the 2S,3S and 2R,3Risomers because they are stereoisomers but not enantiomers. Any given structure can have only one enantiomer. All other stereoisomers of that molecule are diastereomeric. The relative configuration of diastereomeric molecules is frequently specified using the terms synand anti.The molecules are represented as extended chains. Diastereomers with substituents on the same side of the extended chain are synstereoisomers, whereas those with substituents on opposite sides are anti stereoisomers.
Diastereoisomers differ in both physical properties and chemical reactivity. They generally have different melting points, boiling points, solubility, chromatographic mobility, and so on. The specific rotations of diastereomeric molecules differ in both magnitude and sign. The difference in chemical reactivity can be small, such as a difference in rate, or two diastereomers can lead to entirely different products, depending on the mechanism of the particular reaction. Because of their differing physical and chemical properties, diastereomers can be separated by methods such as crystallization or cinematography.
Sometimes the terms erythroand threoare used to specify the relative configuration of two adjacent stereogenic centers. The terms are derived torn the sugars erythrose and threose. The terms were originally defined such that a Fischer projection formula in which two adjacent substituents were on the same side was the erythroisomer and that in which the substituents were on opposite sides was the threoisomer.
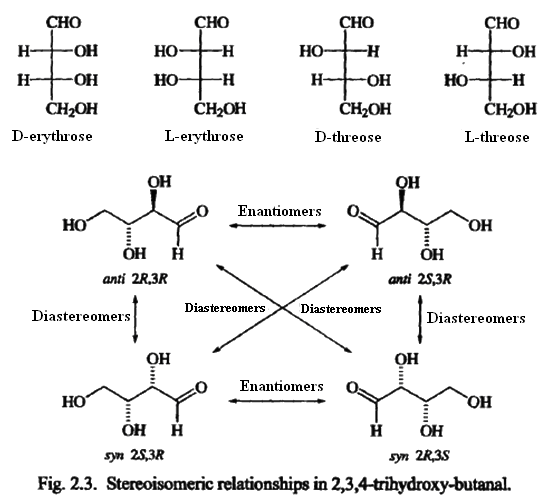
Unfortunately, assignment of molecules that are not closely related to the reference molecules becomes a subjective matter of assigning which substituents are "similar". The application of the terminology to cases in which the chiral centers are not adjacent is also ambiguous. As a result, the threo—erythroterminology is not a general method of specifying stereochemical relationships.
Fischer projection formulas can be used to represent molecules with several stereogenic centers and are commonly used for carbohydrates. For other types of structures, a more common practice is to draw the molecule in an extended conformation with the main chain horizontal In this arrangement, each tetrahedral carbon has two additional substituents, one facing out and one in. The orientation is specified with solid wedged bonds for substituents facing out and with dashed bonds for substituents that point in.
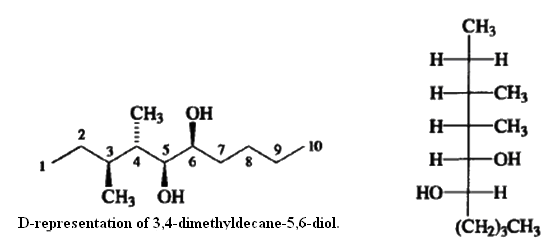
Since the main chain in this representation is in an entirely staggered conformation, whereas in the Fischer projection formulas the conformation represented is completely eclipsed, an antirelationship between two adjacent substituents in an extended structure corresponds to being on the same side in a Fischer projection formula (erythro)whereas a syn relationship corresponds to being on opposite sides in the Fischer projection (threo).
Since chirality is a property of a molecule as a whole, the specific juxtaposition of two or more stereogenic centers in a molecule may result in an achiral molecule. For example, there are three stereoisomers of tartaric acid (2,3-dihydroxybutanedioic acid), Two of these are chiral and optically active but the third is not.The reason that the third stereoisomer is achiral is that the substituents on the two asymmetric carbons are located with respect to each other in such a way that a molecular plane of symmetry exists.Compounds that incorporate asymmetric atoms but are nevertheless achiral are called mesaforms.
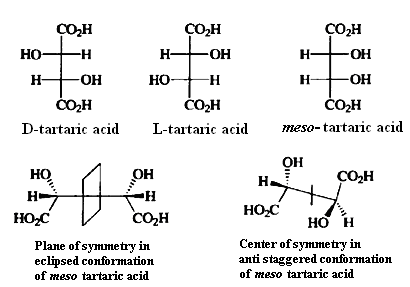
This situation occurs whenever pairs of stereogenic centers are disposed in the molecule in such a way as to create a plane of symmetry. A particularly striking example is the antibiotic nonactin.
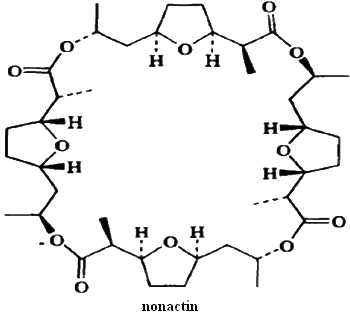
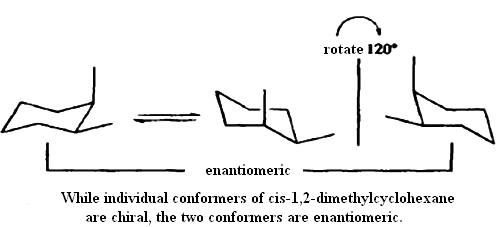
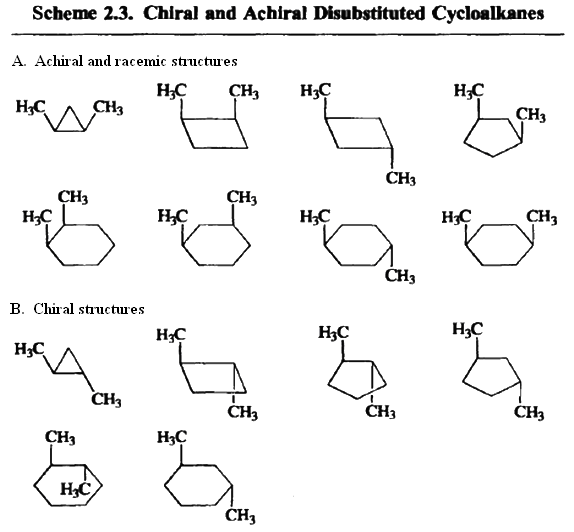
Incorporation of stereogenic centers into cyclic structures produces special stereochemical circumstances. Except in the case of cyclopropane, the lowest-energy conformation of the rings is not planar. Most cyclohexane derivatives adopt a chair conformation. For example, the two conformers of cis-1,2-dimethylcyclohexane are both chiral. However, the two conformers are enantiomeric so the conformational change leads to racemization. Because the barrier to this conformational change is low (10kcal/mol) the two enantiomers are rapidly interconverted.
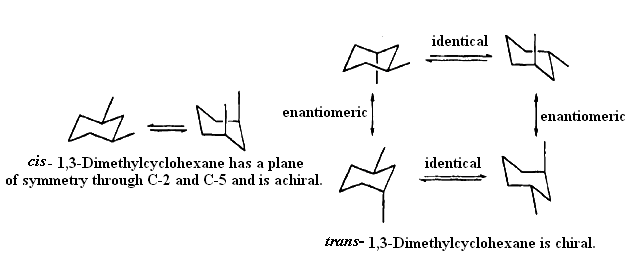
Certain dimethylcycloalkanes contain a plane of symmetry. For example, both chair conformers of cis-1,3-dimethylcyclohexane possess a plane of symmetry bisecting the molecule through C-2 and C-5. The transisomer does not have any element of symmetry and is chiral.
One simple test for chirality of substituted cycloalkanes is to represent the ring in planar form. If the planar form is achiral because of a symmetry element, the compound will not exist as an enantiomerically biased sample, even if individual conformers may be chiral.
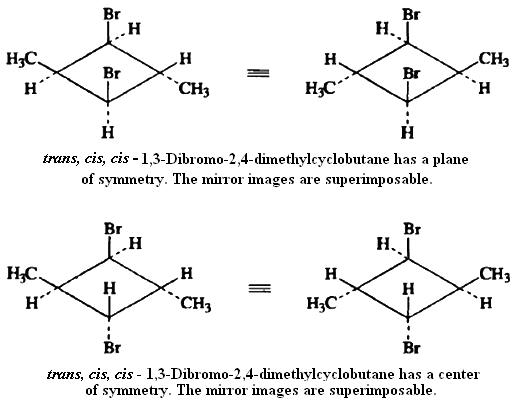
Since the presence of a plane of symmetry in a molecule ensures that it will be achiral, one approach to classification of stereoisomers as chiral or achiral is to examine the molecule for symmetry elements. There are other elements of symmetry in addition to planes of symmetry that ensure that a molecule will be superimposable on its mirror image. The trans, cis, cisand trans, trans, cisstereoisomers of 1,3-dibromo-trans-2,4-dimethylcyclobutane are illustrative, This molecule does not possess a plane of symmetry, but the mirror images are superimposable, as illustrated below. This molecule possesses a center ofsymmetry.A center of symmetry is a point from which any line drawn through the molecule encounters an identical environment in either direction from the center of symmetry.
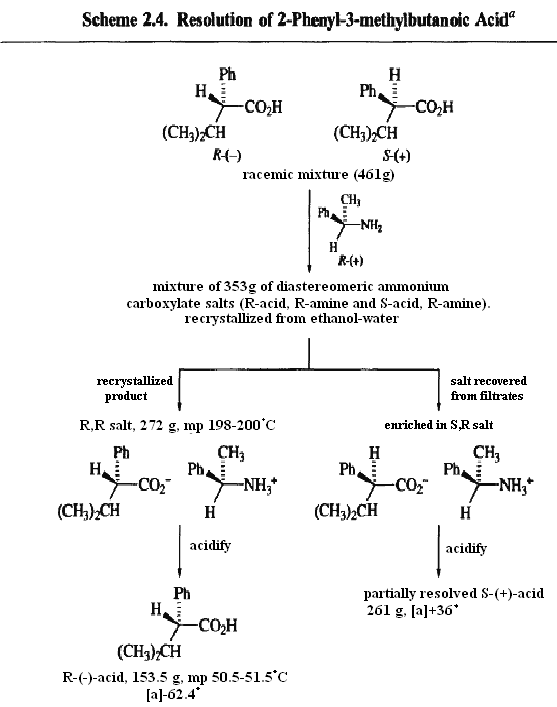
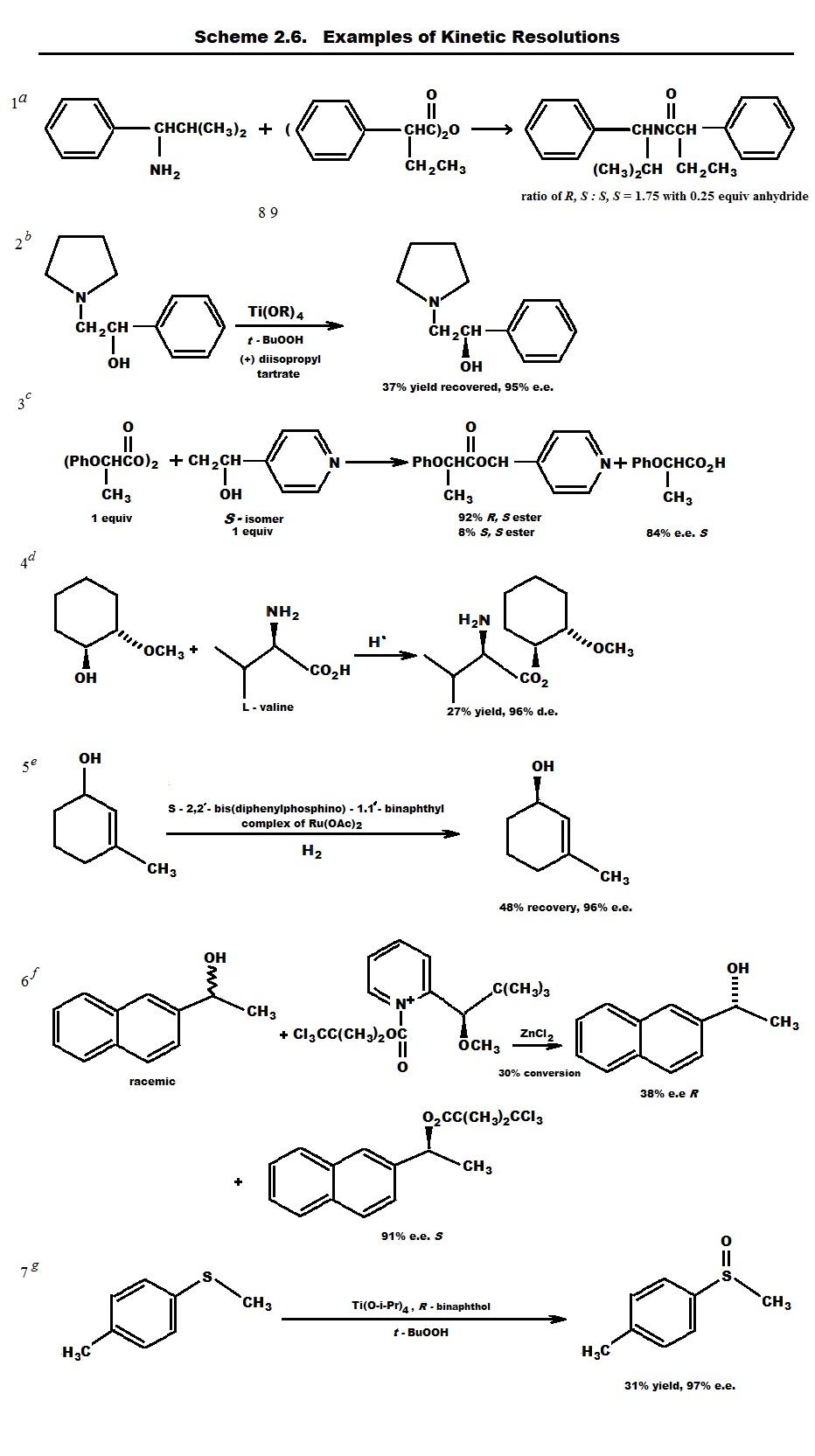
Because diastereoisomers have different physical and chemical properties, they can be separated by a range of chemical and physical methods. The process of resolutionis the separation of a racemic mixture. Separation is frequently effected by converting the enantiomers into a mixture of diastereomers by reaction with a pure enantiomer of a second reagent, the resolving agent.Because the two resulting products will be diastereomeric, they can be separated. The separated diastereomers can then be reconverted to the pure enantiomers by reversing the initial chemical transformation. An example of this method is shown in Scheme for the resolution of a racemic carboxylic acid by way of a diastereomeric salt resulting from reaction with an enantiomerically pure amine. The R-acid, R-amine and S-acid, S-amine salts are separated by fractional recrystallization. The resolved acids are regenerated by reaction with a strong acid, which liberates the carboxylic acid from the amine salt.
Although the traditional method of separating the diastereomeric compounds generated in a resolution procedure is fractional crystallization, chromatographic procedures are now common and convenient. Diastereomeric compounds exhibit different adsorption on achiral materials and can be separated by column chromatography or by taking advantage of the greater separation powers of HPLC.
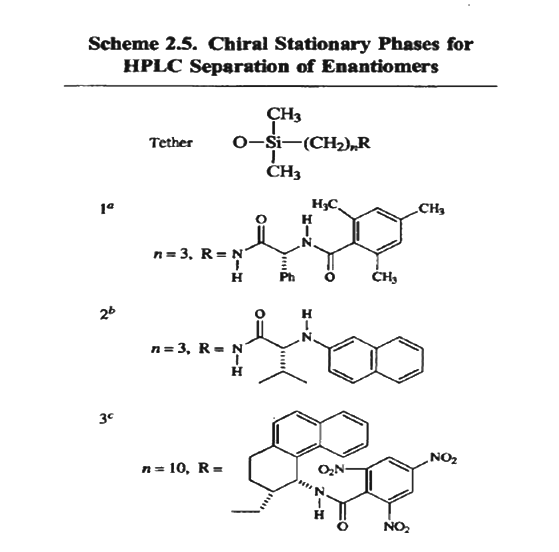
Separation of enantiomers by physical or chemical methods requires the use of a chiral material, reagent, or catalyst. Both natural materials, such as polysaccharides and proteins, and solids that have been synthetically modified to incorporate chiral structures have been developed for use in separation of enantiomers by HPLC. The use of a chiral stationary phase makes the interactions between the two enantiomers with the adsorbent nonidentical and thus establishes a different rate of elution through the column. The interactions typically include hydrogen bonding, dipolar interactions, and π-π interactions. These attractive interactions may be disturbed by steric repulsions, and frequently the basis of enantioselectivity is a better steric fit for one of the two enantiomers.
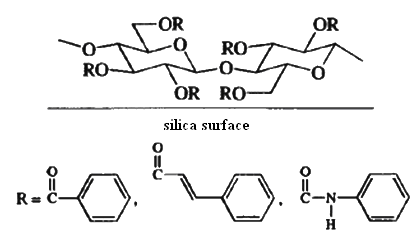
The potential for use of chiral natural materials such as cellulose for separation of enantiomers has long been recognized, but development of efficient materials occurred relatively recently. Several acylated derivatives of cellulose are effective chiral stationary phases. Benzoate esters and aryl carbamates are particularly useful. These materials are commercially available on a silica support and under the trademark Chiralcel.
Synthetic chiral adsorbents are usually prepared by tethering a chiral molecule to a silica surface. The attachment to the silica is through alkylsiloxy bonds. A study which demonstrates the technique reports the resolution of a number of aromatic compounds on a 1- to 8-g scale. The adsorbent is a silica that has been derivatized with a chiral reagent. Specifically, hydroxyl groups on the silica surface are covalently bound to a derivative of R-phenylglycine.
A medium-pressure chromatography apparatus is used. The racemic mixture is passed through the column, and, when resolution is successful, the separated enantiomers are isolated as completely resolved fractions.
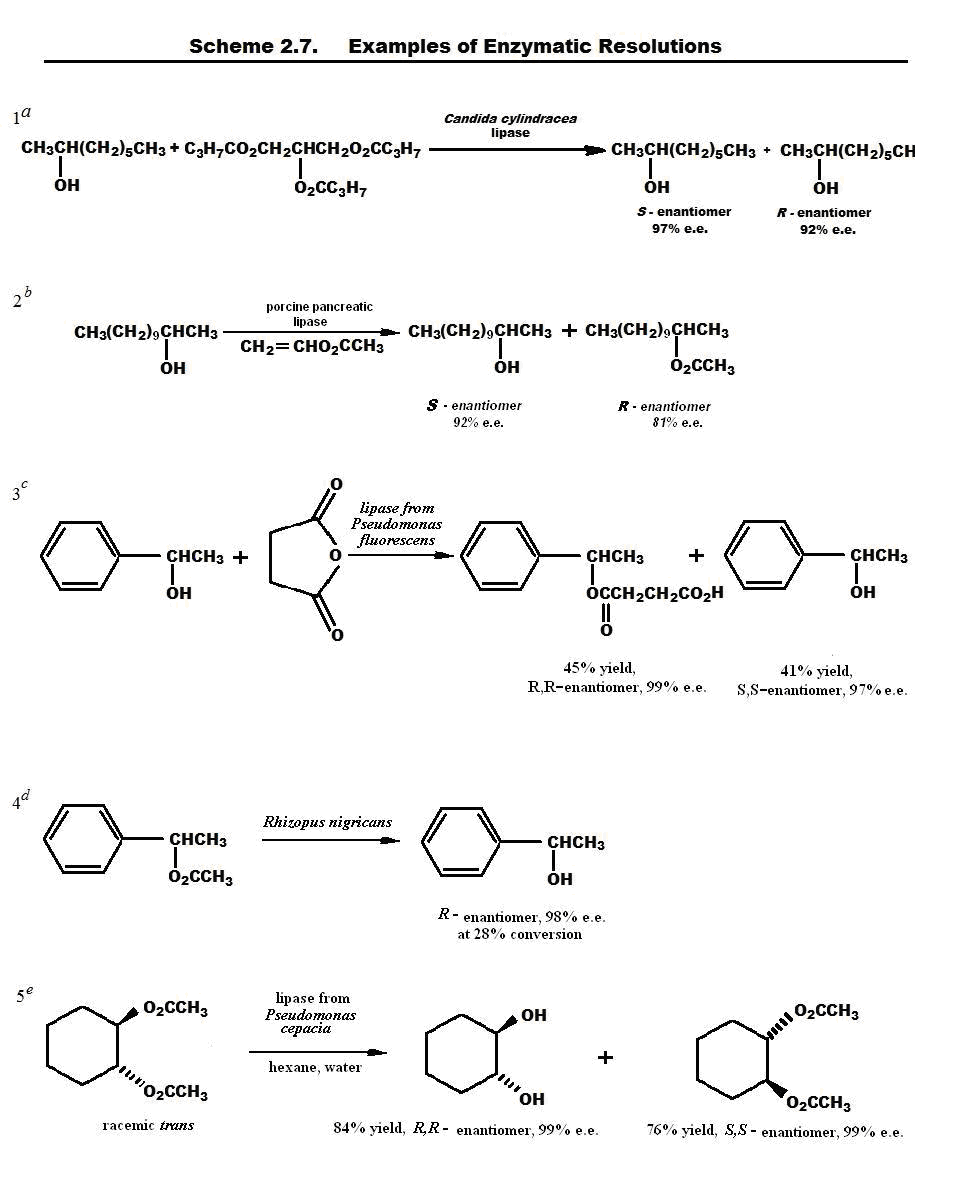
Another means of resolution depends on the difference in rates of reaction of two enantiomers with a chiral reagent. The transition-state energies for reaction of each enantiomer with one enantiomer of a chiral reagent will be different. This is because the transition states and intermediates (R-substrate… R-reactant) and (S-substrate…S-reactant) are diastereomeric. Kinetic resolutionis the term used to describe the separation of enantiomers based on different reaction rates with an enantiomerically pure reagent.
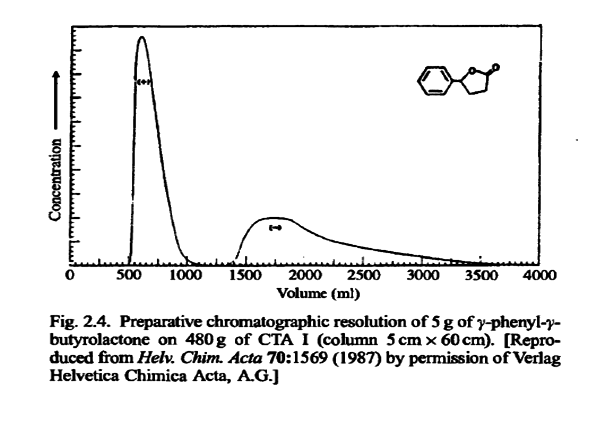
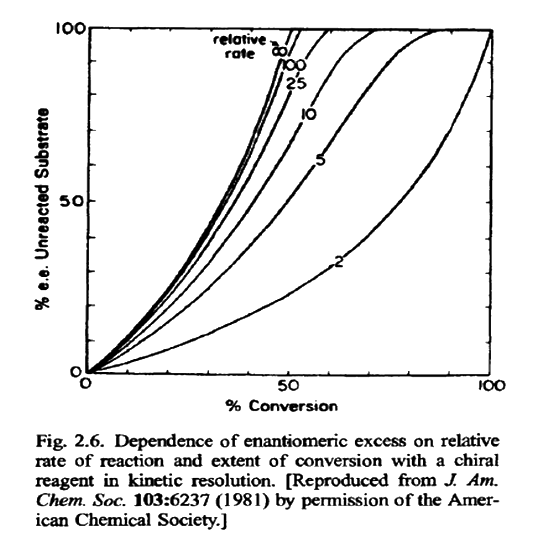
Figure 2.5 summarizes the basis of kinetic resolution. Because the separation is based on differing rates of reaction, the degree of resolution that can be achieved depends on both the magnitude of the rate difference and the extent of reaction. The greater the difference in the two rates, the higher the enantiomeric purity of both the reacted and the unreacted enantiomer. The extent of enantiomeric purity can be controlled by controlling the degree of conversion. As the degree of conversion increases, the enantiomeric purity of the unreacted enantiomer becomes very high.
The relationship between the relative rate of reaction, extent of conversion, and enantiomeric purity of the unreacted enantiomer is shown in Fig. 2.6. Of course, the high conversion required for high enantiomeric purity when the relative reactivity difference is low has a serious drawback. The yieldof the unreactedsubstrate is low if the overall conversion is high Thus, with relative reactivity differences of < 10, high enantiomeric purity can be achieved only at the expense of low yield.
Preparation of enantiomerically enriched materials by use of chiral catalysts is also based on differences in transition-state energies. While the reactant is part of a complex or intermediate containing a chiral catalyst, it is in a chiral environment The intermediates and complexes containing each enantiomeric reactant and a homochiral catalyst are diastereomeric and differ in energy, This energy difference can then control selection between the stereoisomeric products of the reaction. If the reaction creates a new stereogenic center in the reactant molecule, there can be a preference for formation of one enantiomer over the other.
Enzymes constitute a particularly important group of enantioselective catalysts. Enzymes are highly efficient and selective catalysts and can carry out a variety of transformations. Because the enzymes are derived from L-amino acids, they are homochiral, and usually one enantiomer of a reactant is much more reactive than the other. The reason is that the interaction of the enzyme with one enantiomer is diastereomeric to its interaction with the other. Because enzyme catalysis is usually based on a specific fit to an "active site," the degree of selection between the two enantiomers is often very high. Enzyme-catalyzed reactions can therefore be used to resolve organic compounds. The most completely characterized enzymes that are available are those which catalyze hydrolysis of esters and amides (esterases, lipases, peptidases, acylases) and those which oxidize alcohols to ketones or aldehydes (dehydrogenases). Purified enzymes can be used, or the reaction can be done by incubating the reactant with an organism (yeast, for example) that produces an appropriate enzyme during fermentation.
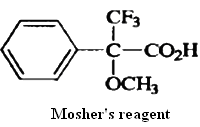
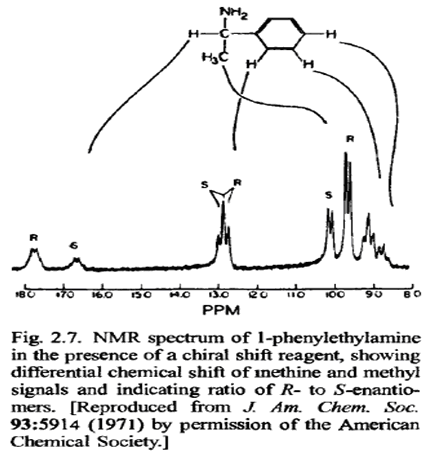
The differing physical properties of diastereomers are also the basis for a particularly sensitive method for assessing the enantiomeric purity of compounds. Although, in principle, enantiomeric purity can be determined by measuring the optical rotation, this method is reliable only if the rotation of the pure compound is accurately known. This is never the case for a newly prepared material and is often uncertain for previously prepared compounds. If a derivative of a chiral compound is prepared in which a new chiral center is introduced, the two enantiomers will give different diastereomers. Because these will have different physical properties, their relative amounts can be determined. NMR spectroscopy is a convenient means of detecting and quantitating the two diastereomeric products. A pure enantiomer will give only a single spectrum, but a partially resolved material will show two overlapping spectra in the ratio of the two diastereomeric derivatives. The most widely used derivatizing reagent for the NMR method is a compound known as Mother's reagent.One reason that this compound is particularly useful is mat the aromatic ring usually induces markedly different chemical shifts in the two diastereomeric products that are formed.
Changes in NMR spectra can also be observed as the result of formation of noncovalent complexes between enantiomeric molecules and another chiral reagent. This is the basis of the use of chiral shift reagentsto determine the enantiomeric purity of chiral substances. Several of the lanthanide elements have the property of forming strong complexes with alcohols, ketones, and other functional groups having Lewis base character. Ifthe lanthanide ion is in a chiral environment as the result of an enantiomerically pure ligand, two diastereomeric complexes are formed. The lanthanide elements induce large NMR shifts, and, as a result, shifted spectra are seen for the two complexed enantiomers. The relative intensities of the two spectra correspond to the ratio of enantiomers present in the sample.
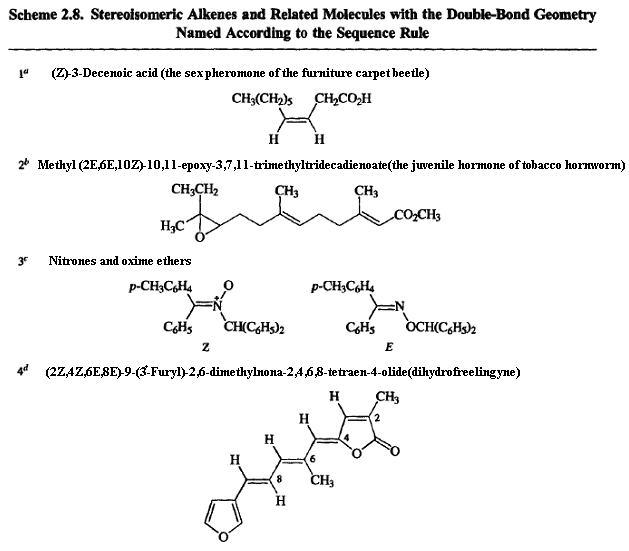
Geometric isomers of alkenes are diastereomeric, since they are stereoisomers but not enantiomeric. The specification of the geometry of double bonds as cisand transsuffers from the same ambiguity as specifying configuration by the Fischer convention; that is, it requires a subjective judgment about the "similarity'" of groups. The sequence rule is the basis for an unambiguous method for assignment of alkene geometry. The four substituents on the double bond are taken in pairs. The sequence rules are used to determine if the higher-priority groups on the atoms forming the double bond are on the same side or opposite sides of the double bond.
If the higher-priority groups are on the same side, the descriptor is Z(from the German word zusammen,together); if they are on opposite sides, the descriptor is E(from entgegen,opposite). As in applying the sequence rule to stereogenic centers, if the atoms directly attached to the double bond have the same atomic number, the priorities are assigned by sequentially comparing atoms in the substituent until priority can be established. The system can also be applied to multiple bonds involving elements other than carbon, such as C=N. The Zand Edescriptors have replaced synand antifor describing the stereochemistry of oximes. As in the case of stereogenic centers, if an atom at a double bond does not have two substituents (as is the case for oximes), then a "phantom ligand" with atomic, number zero is assumed and assigned the lower priority. Scheme 2.8 shows some stereoisomers compounds named according to the sequence rule convention.