- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
Principles of Stereochemistry
For most combinations of atoms, a number of molecular structures that differ from each other in the sequence of bonding of the atoms are possible. Each individual molecular assembly is called an isomer, and the constitution of a compound is the particular combination of bonds between atoms (molecular connectivity) which is characteristic of that structure. Propanal,allyl alcohol, acetone, 2-methyloxirane, and cyclopropanol each correspond to the molecular formula C3H6O, but differ in constitution and are isomers of one another.
When structures having the same constitution differ with respect to their spatial arrangement, they are stereoisomers.Stereoisomers are described by specifying their topology and the nature of their relationship to other stereoisomers of the same constitution. Stereoisomers differ in configuration,and in order to distinguish between stereoisomeric compounds, it is necessary to specify the configuration. If two stereoisomers are nonsuperimposable mirror images, the molecules are enantiomers. Structures which have nonsuperimposable mirror images are called chiral. Chirality is the property of any molecule (or other object) of being nonsuperimposable on its mirror image. Samples which contain only one enantiomer are called enantiomerically pure or homochiral. Stereoisomers which are not enantiomers are diastereomers.
It is possible to obtain pure enantiomers of chiral compounds. One property of separated enantiomers is to cause the rotation of the plane of polarized fight by oppositebut equal amounts. Samples mat contain equal amounts of two enantiomers have zero net rotation and are called racemic mixtures. Samples that contain only one of the enantiomers are said to be enantiomerically pure. Samples that have an excess of one enantiomer over the other are enantiomerically enriched and show a net rotation of polarized light and are said to be optically active.
In addition to constitution and configuration, there is a third important level of structure, that of conformation. Conformations are discrete molecular arrangements that differ in spatial arrangement as a result of facile rotations about single bonds. Usually, conformers are in thermal equilibrium and cannot be separated. A special case of stereoisomerism arises when rotation about single bonds is sufficiently restricted by steric or other factors that-the different conformations can be separated. The term atropisomeris applied to stereoisomers that result from restricted bond rotation.
Enantiomeric Relationships
The relationship between chirality and optical activity is historically such a close one that chemists sometimes use the terms imprecisely. Optical activity refers to just one property of chiral molecules, namely, the ability to rotate plane-polarized light Measurement of optical activity is useful both for determining the configuration of chiral molecules and for investigating the stereochemical relationship between reactants and products. The mechanics of measuring optical rotation will not be discussed here since the basic method is described in most introductory texts. Both the sign and the magnitude of optical rotation are dependent on the conditions of the measurement, including temperature, solvent, and the wavelength of the light By convention, single-wavelength measurements are usually made at the 589-nm emission line of sodium arc lamps. This wavelength is known as the sodium D line, and optical rotations measured at this wavelength are designated [ɑ]D.
Pure enantiomeric substances show rotations that are equal in magnitude but opposite in direction. Unequal mixtures of enantiomers rotate light in proportion to the composition. The relationship between optical purity and measured rotation is
Optical
purity (%) = ![]()
![]()
The optical purity is numerically equivalent to the enantiomeric excess (e.e.), which is defined as
![]()
Measurement of rotation as a function of wavelength is useful in structural studies aimed at determining the chirality of a molecule. This technique is called optical rotatory dispersion (ORD). The resulting plot of rotation against wavelength is called an ORD curve. The shape of the ORD curve is determined by the configuration of the molecule and its absorption spectrum. In many cases, the ORD curve can be used to specify the configuration of a molecule by relating it to those of similar molecules of known configuration. Chiral substances also show differential absorption of circularly polarized light This is called circular dichroism(CD) and is quantitatively expressed as the molecular ellipticity, Ө:
![]()
where ԑL and ԑRare the extinction coefficients of left and right circularly polarized light.
The molecular ellipticity is analogous to specific rotation in that two enantiomers have exactly opposite values of Өat every wavelength. Two enantiomers will thus show CD spectra having opposite signs. A compound with several absorption bands may show both positive and negative bands.
Although measurements of optical rotation and ORD or CD spectra have historically been the main methods for determining enantiomeric purity and assisting configuration, other analytical techniques are also available. High-performance liquid chromatography (HPLC) using chiral column packing material can resolve enantiomers on both an analytical and a preparative scale. Chiral packing materials for gas-liquid chromatography (GLC) have also been developed.
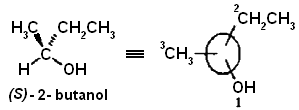
Compounds in which one or more carbon atoms have four nonidentical substituents are the largest class of chiral molecules, Carbon atoms with four nonidentical ligands are referred to as asymmetric carbon atoms because the molecular environment at such a carbon atom possesses no element of symmetry. Asymmetric carbons are a specific example of a stereogenic center. A stereogenic center is any structural feature that gives rise to chirality in a molecule. 2-Butanol is an example of a chiral molecule and exists as two nonsuperimposable mirror images. Carbon-2 is a stereogenic center.
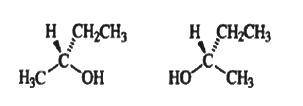
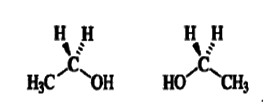
Ethanol is an achiral molecule. The plane defined by atoms C-1,C-2, and O is a plane of symmetry. Any carbon atom with two identical ligands contains a plane of symmetry that includes the two nonidentical ligands. Any molecule, no matter how complex, that possesses a plane of symmetry is achiral.
There are a number of important kinds of stereogenic centers besides asymmetric carbon atoms. One example is furnished by sulfoxides with nonidentical substituents on sulfur. Sulfoxides are pyramidal and maintain their configuration at room temperature. Unsymmetrical sulfoxides are therefore chiral and exist as enantiomers. Sulfonium salts with three nonidentical ligands are also chiral as a result of their pyramidal shape.
Although unsymmetrically substituted amines are chiral, the configuration is not stable because of rapid inversion at nitrogen. The activation energy for pyramidal inversion at phosphorus is much higher than at nitrogen, and many optically active phosphines have been prepared. The barrier to inversion is usually in the range of 30-35 kcal/mol so that enantiomerically pure phosphines are stable at room temperature but racemize by inversion at elevated temperatures. Asymmetrically substituted tetracoordinate phosphorus compounds such as phosphonium sails and phosphine oxides are also chiral.
The chirality of a molecule is described by specifying its configuration. The system that is used is the Cahn-Ingold-Prelogconvention, which uses the descriptors Rand S.The Fischer convention,employing the descriptors D and L, is historically important and is still used with certain types of molecules.
The Cahn Ingold-Prelog descriptors Rand Sare assigned by using the sequence rideto assign a priority order to the substituents on the atom to which a configuration is being assigned The substituent atoms are assigned decreasing priority in the order of decreasing atomic number. When two or more of the substituent atoms are the same dement (e.g., carbon), the assignment of priority is based on the next attached atom in those substituents. This process is continued until the order of priority of all substituents has been established. An atom that is multiply bonded is counted once for each formal bond. When the substituent priority has been established, the molecule is viewed in an orientation that places the lowest-priority substituent behind the stereogenic center. The three remaining substituents project toward the viewer. The remaining substituents have one of two possible arrangements. The substituents decrease in priority in either a clockwise manner or in a counterclockwise manner. In the former case, the configuration R(for rectus)is assigned. If the priority decreases in the counterclockwise sense, the atom is of S (for sinister)configuration.
The configuration of the 2-butanol enantiomer shown below is established as Sas follows. The highest-priority atom bonded to the asymmetric carbon is O; the lowest is H. The remaining two atoms are each C, and the choice as to which of these is of higher priority is made by comparing their ligands. The methyl group has (H, H, H), while the ethyl group has (С, H, H); therefore, the ethyl group is of higher priority than the methyl group. The complete priority list is; OH > C 2H 5> CH3> H. When viewed from the side opposite the lowest-priority ligand, the remaining groups appear in order of decreasing priority in counterclockwise fashion, and the configuration is S
Some other examples of assignment of configuration are illustrated below.
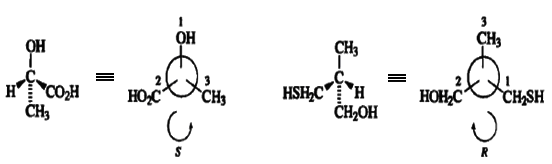
When a stereogenic center is tricoordinate, as is the case for sulfoxides, sulfonium salts, and phosphines, then a "phantom atom" of atomic number zero is taken to occupy the lowest-priority site of a presumed tetrahedral atom.
Glyceraldehye is the point of reference for describing the configuration of carbohydrates and other natural substances in accordance with the Fischer convention.The two enantiomers were originally arbitrarily assigned the configurations d and l as shown below. Subsequently, a determination of the configuration of sodium rubidium tartrate by X-ray crystallography and the relationship of this material to D-glyceraldehyde established that the original arbitrary assignments were the correct ones.
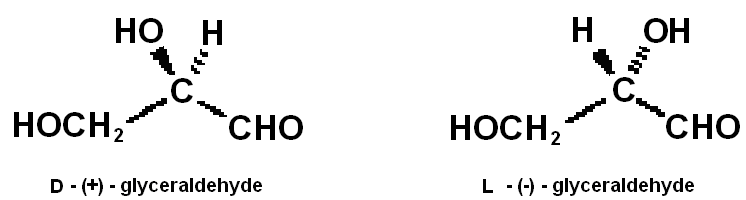
In the Fischer convention, the configuration is of other molecules are described by the descriptors d and l, which are assigned by comparison with the reference molecule glyceraldehyde. In employing the Fischer convention, it is convenient to use projection formulas.These are planar representations Defined in such a way as to convey three-dimensional structural information. The molecule is oriented with the major carbon chain aligned vertically in such a manner mat the most oxidized terminal carbon is at the top. The vertical bonds at each carbon are directed back, away from the viewer, and the horizontal bonds are directed toward the viewer. The D and L forms of glyceraldehyde are shown below with the equivalent Fischer projection formulas.
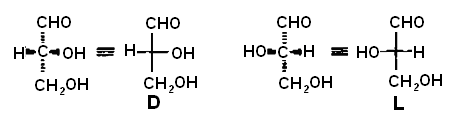
The assignment of the configuration of any other chiral molecule in the Fischer convention is done by comparison with D- and L-glyceraldehyde. The molecule is aligned with the chain vertical and the most oxidized carbon at the top, as specified by the Fischer convention. The stereogenic center with the highest number (at the lowest position in the Fischer projection) is compared with C-2of glyceraldehyde. If the configuration is that of D-glyceraldehye, the molecule is assigned the D-configuration, whereas if it is like that of L-glyceraldehyde, it is assigned the L-configuration. This is illustrated below with several carbohydrates.
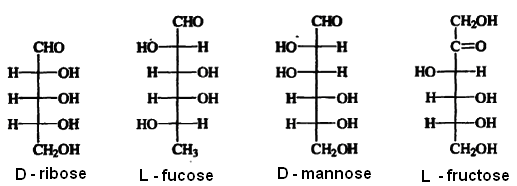
The amino acids found in proteins have the L-configuration, as illustrated for alanine, serine, and leucine.
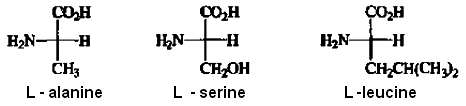
At the present time, use of the Fischer convention is almost entirely restricted to carbohydrates, amino acids, and biologically important molecules of closely related structural types. The problem with more general use is that there are no adequate rules for deciding whether a chiral atom is "tike" D-glyceraldehyde or L-glyceraldehyde when the structures are not closely similar to the reference molecules. This relationship is clear for carbohydrates and amino acids.
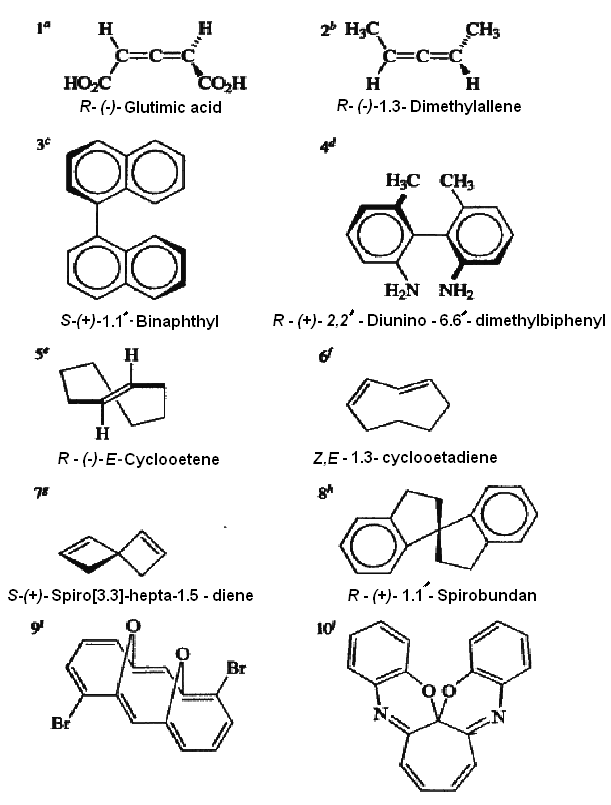
The property of chirality is determined by overall molecular topology, and there are many molecules that are chiral even though they do not possess an asymmetrically substituted atom. The chirality of E-cyclooctene and Z,E-cyclooctadiene is also dependent on restricted rotation. Manipulation of a molecular model will illustrate that each of these molecules can be converted into its enantiomer by a rotational process by which the ring is turned "inside-out."
There is no direct relationship between the configurational descriptors R and S or D and l and the sign of rotation of the molecule. Ror Smolecules can have either + or - signs for rotation, as can d or L molecules. Thus, even though a configuration can be specified on the basis of these conventions, additional information is necessary to establish which molecule of an enantiomeric pair possesses the specified configuration. Determination of the absolute configurationestablishes the configuration of each enantiomer. There are several approaches to this problem. One is to establish a direct structural relationship to a molecule of known configuration by chemical transformation. This is the way in which most of the reference molecules whose absolute configurations are known were initially assigned. The existence of a base of molecules whose absolute configurations are known has permitted the development of correlations based on the CD and ORD curves of certain types of chromophores. When chromophores are located close to stereogenic centers, the spectroscopic properties are affected in a predictable way so that the sign and shape of the ORD or CD curve can be a reliable basis for configurational assignment. While routine X-ray crystal structure determination does not provide the absolute configuration of the molecule, special analysis of the diffraction data does allow assignment of absolute configuration. These methods are important, for example, in assigning the absolute configuration of new natural products.