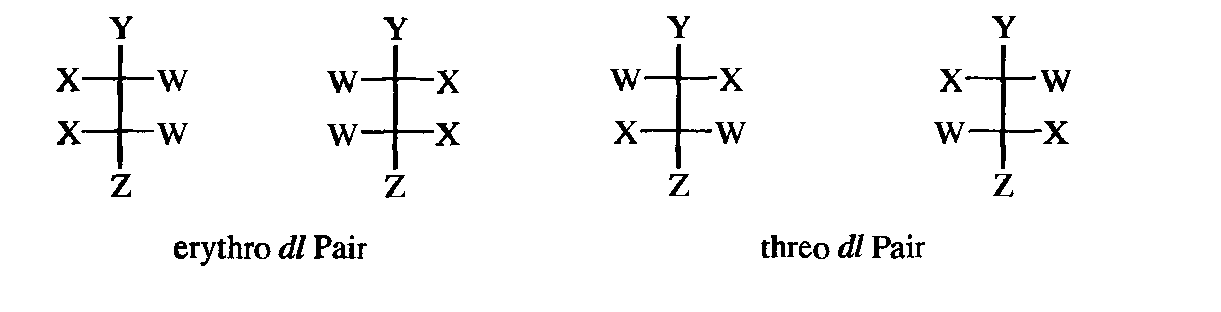- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
Molecules With More Than One Chiral (Stereogenic) Center
When a molecule has two stereogenic centers, each has its own configuration and can be classified (R)or (S) by the Cahn-Ingold-Prelog method. There are a total of four isomers, since the first center may be (R)or (S)and so may the second. Since a molecule can have only one mirror image, only one of the other three can be the enantiomer of A. This is B [the mirror image of an (R)center is always an (S) center]. The compounds C and D are a second pair of enantiomers and the
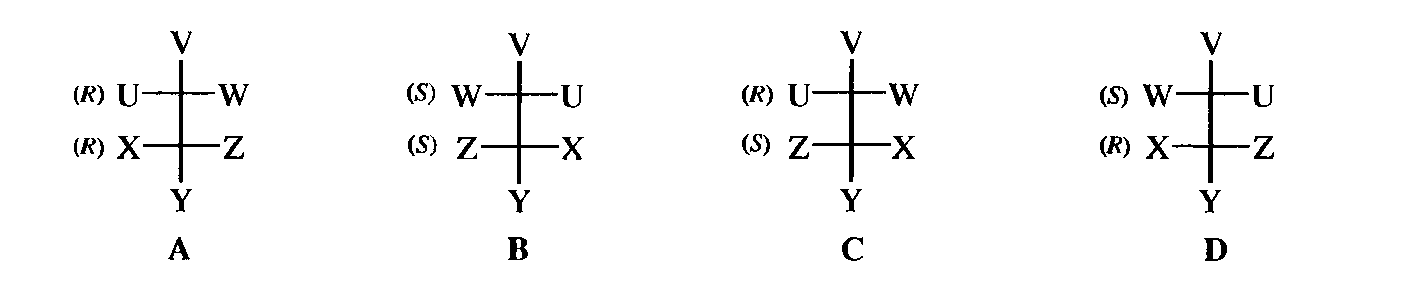
relationship of C and D to A and B is designated by the term diastereomer. Diastereomers may be defined as stereoisomers that are not enantiomers. Being enantiomers, C and D must have identical properties; the same is true for A and B. However, the properties of A and B are not identical with those of C and D. They have different melting points, boiling points, solubilities, reactivity, and all other physical, chemical, and spectral properties. The properties are usually similar but not identical. In particular, diastereomers have different specific rotations; indeed one diastereomer may be chiral and rotate the plane of polarized light while another may be achiral and not rotate at all (an example is presented below).
It is now possible to see why enantiomers react at different rates with other chiral molecules but at the same rate with achiral molecules. In the latter case, the activated complex formed from the (R)enantiomer and the other molecule is the mirror image of the activated complex formed from the (S)enantiomer and the other molecule. Since the two activated complexes are enantiomeric, their energies are the same and the rates of the reactions in which they are formed must be the same. However, when an (R)enantiomer reacts with a chiral molecule that has, say, the (R)configuration, the activated complex has two chiral centers with configurations (R)and (R),while the activated complex formed from the (S)enantiomer has the configurations (S)and (R). The two activated complexes are diastereomeric, do not have the same energies, and consequently are formed at different rates.
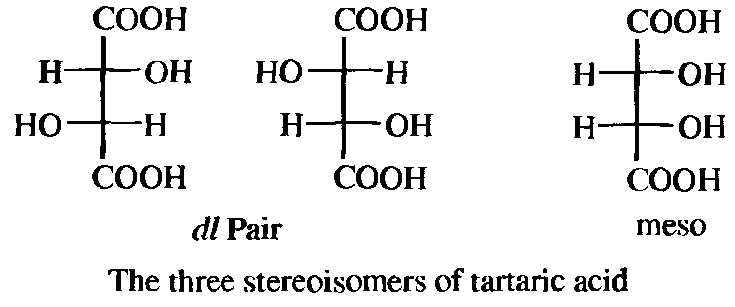
Although four is the maximum possible number of isomers when the compound has two chiral centers (chiral compounds without a chiral carbon, or with one chiral carbon and another type of chiral center, also follow the rules described here), some compounds have fewer. When the three groups on one chiral atom are the same as those on the other, one of the isomers (called a meso form) has a plane of symmetry, and hence is optically inactive, even though it has two chiral carbons. Tartaric acid is a typical case. There are only three isomers of tartaric acid: a pair of enantiomers and an inactive meso form. For compounds that have two chiral atoms, meso forms are found only where the four groups on one of the chiral atoms are the same as those on the other chiral atom.
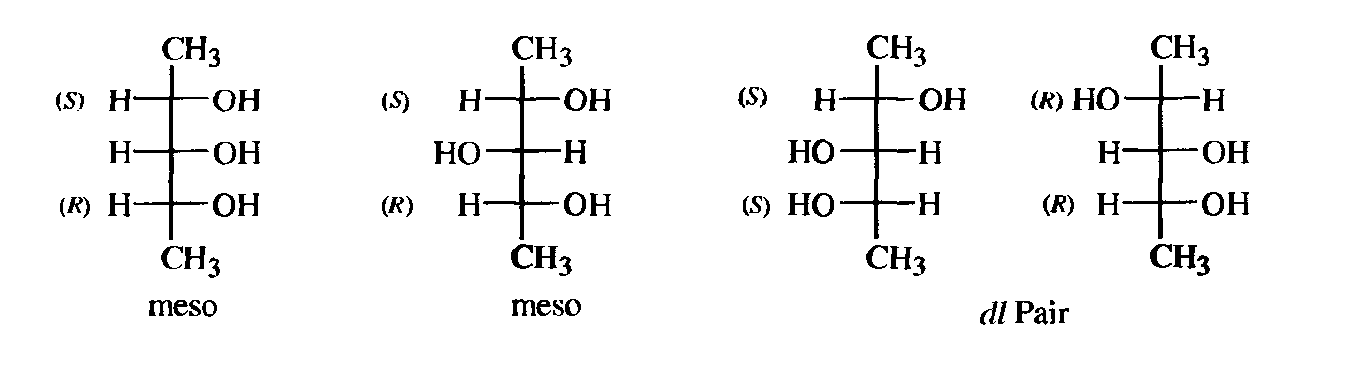
In most cases with more than two chiral centers, the number of isomers can be calculated from the formula 2n where n is the number of chiral centers, although in some cases the actual number is less than this, owing to meso forms. An interesting case is that of 2,3,4-pentanetriol (or any similar molecule). The middle carbon is not asymmetric when the 2- and 4-carbon atoms are both (R)[or both (S)] but is asymmetric when one of them is (R)and the other (S).Such a carbon is called a pseudoasymmetric carbon. In these cases, there are four isomers: two meso forms and one dl pair. The student should satisfy himself or herself, remembering the rules governing the use of the Fischer projections, that these isomers are different, that the meso forms are superimposable on their mirror images, and that there are no other stereoisomers. Two diastereomers that have a different configuration at only one chiral center are called epimers.
In compounds with two or more chiral centers, the absolute configuration must be separately determined for each center. The usual procedure is to determine the configuration at one center, and then to relate the configuration at that center to the others in the molecule. One method is X-ray crystallography, which cannot be used to determine the absolute configuration at any chiral center but which does give relative configurations of all the chiral centers in a molecule, and hence the absolute configurations of all once the first is independently determined. Other physical and chemical methods have also been used for this purpose.
The problem of how to name the different stereoisomers of a compound when there are more than two now arises. Enantiomers are virtually always called by the same name, being distinguished by (R)and (S) or d and l or ( + ) and (-). In the early days of organic chemistry, it was customary to give each pair of enantiomers a different name or at least a different prefix (such as epi-, peri-, etc.). Thus the aldohexoses are called glucose, mannose, idose, and so on, although they are all 2,3,4,5,6-pentahydroxyhexanal (in their open-chain forms). This practice was partially due to lack of knowledge about which isomers had which configurations. Today it is customary to describe each stereogenic position separately as either (R) or (S) or, in special fields, to use other symbols. Thus, in the case of steroids, groups above the "plane" of the ring system are designated β, and those below it ɑ. Solid lines are often used to depict βgroups and dashed lines for ɑ groups. An example is

For many open-chain compounds, prefixes are used that are derived from the names of the corresponding sugars and that describe the whole system rather than each chiral center separately. Two such common prefixes are erythro- and threo-, which are applied to systems containing two stereogenic carbons when two of the groups are the same and the third is different. The erythro pair has the identical