
- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
Unit 5 resolution of enantiomers
Reading
Section A
You are going to read the text about resolution of enantiomers. Before reading answer the following questions:
What isLouis Pasteur famous for? What was he investigating?
What is Fischer projection?
What do you know aboutvan't Hoff and Le Bel? Who are they? What are they famous for?
Skim the text and be able to answer the questions:
- what does this text deal with?
- what is the subject of the information?
- what are the main aspects of the text?
Resolution of enantiomers
The separation of a racemic mixture into itsenantiomeric components is termed resolution. The first resolution, that of tartaric acid, was carried out by Louis Pasteur in 1848. Tartaric acid is a byproduct of wine making and is almost always found as its dextrorotatory 2R,3R stereoisomer, shown here in a perspective drawing and in a Fischer projection.
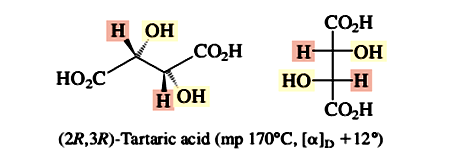
Occasionally, an optically inactive sample of tartaric acid was obtained. Pasteur noticed that the sodium ammonium salt of optically inactive tartaric acid was a mixture of two mirror-image crystal forms. With microscope and tweezers, Pasteur carefully separated the two. He found that one kind of crystal (in aqueous solution) was dextrorotatory, whereas the mirror-image crystals rotated the plane of polarized light an equal amount but were levorotatory.
Although Pasteur was unable to provide a structural explanation—that had to wait for van't Hoff and Le Bel a quarter of a century later—he correctly deduced that the enantiomeric quality of the crystals was the result of enantiomeric molecules. The rare form of tartaric acid was optically inactive because it contained equal amounts of (+)-tartaric acid and (-)-tartaric acid. It had earlier been called racemic acid (from Latin racemus, a “ bunch of grapes”), a name that subsequently gave rise to our present term for an equal mixture of enantiomers.
Pasteur's technique of separating enantiomers not only is laborious but requires that the crystal habits of enantiomers be distinguishable. This happens very rarely. Consequently, alternative and more general approaches for resolving enantiomers have been developed. Most are based on a strategy of temporarily converting the enantiomers of a racemic mixture to diastereomeric derivatives, separating these diastereomers, then regenerating the enantiomeric starting materials.
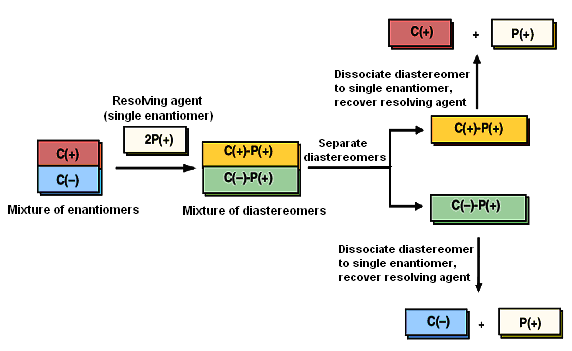
Figureillustrates this strategy. Say we have a mixture of enantiomers, which, for simplicity, we label as C( + ) and C(-). Assume that C(+) and C(-) bear some functional group that can combine with a reagent P to yield adducts C( + )-P and C(-)-E. Now, if reagent P is chiral, and if only a single enantiomer of P, say, P(+), is added to a racemic mixture of C(+) and C(-), as shown in the first step of Figure, then the products of the reaction are C(+)-P(+) and C(-)-P(+). These products are not mirror images; they are diastereomers. Diastereomers can have different physical properties, which can serve as a means of separating them. The mixture of diastereomers is separated, usually byrесrуstallization from a suitable solvent. In the last step, an appropriate chemical transformation liberates the enantiomers and restores the resolving agent.
Whenever possible, the chemical reactions involved in the formation of diastereomers and their conversion to separate enantiomers are simple acid-base reactions. For example, naturally occurring (S)-(-)-malic acid is often used to resolve amines. One such amine that has been resolved in this way is 1-enylethylamine. Amines are bases, and malic acid is an acid. Proton transfer from (S)-(-)-malic acid to a racemic mixture of (R)- and (S)-1-phenylethylamine gives a mixture of diastereomeric salts.
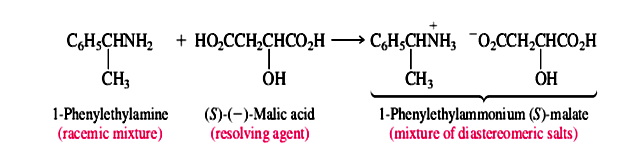
The diastereomeric salts are separated and the individual enantiomers of the amine liberated by treatment with a base:
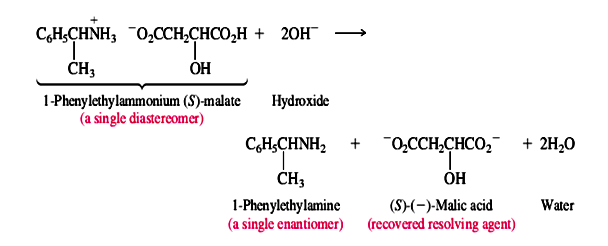
This method is widely used for the resolution of chiral amines and carboxylic acids. Analogous methods based on the formation and separation of diastereomers have been developed for other functional groups; the precise approach depends on the kind of chemical reactivity associated with the functional groups present in the molecule.
The rapidly increasing demand for enantiomerically pure starting materials and intermediates in the pharmaceutical industry has increased interest in developing methods for resolving racemic mixtures.
Read the text once more identify introductory, main and conclusion parts. Express the main idea of each part in one sentence. What is the author’s conclusion of the text?
Based on the theme of the text, write down five sentences starting with the phrase “One thing I really don’t understand is…”. Share and compare the sentences with your partner. Try to enlighten each other regarding the things you don’t understand.
Render the text.
Section B
