
- •Англійська мова для професійного спілкування
- •Передмова
- •Brief contents
- •Unit 1 structure and bonding
- •1. You are going to read three texts which are all connected with chemistry. Read the texts and be able to make intelligent guesses about:
- •2. Decide what books the texts come from. What helped you to make up your mind? Choose from the following:
- •3. Which sentence could be the opening sentence of the text?
- •4. Think about the first sentences above and decide which you think are likely to introduce a paragraph with:
- •6. Give the definitions of the following terms:
- •2. Look at Appendix 3 and Render the following text.
- •3. Read the following text. Discuss the point with your colleagues. What do you know about the methods of scientific investigation? The Scientific Method
- •The Scientific Method
- •1. Culture clips: London life
- •2.What museums are there in your city/town? Have you ever visited any?
- •3.Have you ever visited science museum of the “kpi”? Are there any in your university? Imagine that you are a guide at such museum, tell about the most interesting museum piece.
- •2. What was said in the text about:
- •3. Render the following text.
- •1. Imagine that you are starting a presentation. What phrases might you use?
- •2. Listen totwowaysofopeningpresentationsandseeifyoucanhearsomeofthephrasesabove.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own opening for the topic “Stereochemistry”.
- •Imagine that you are a major distributor of the following product. Look at Business English section and write a letter asking more information about the product presented below.
- •Unit 3 molecular symetry
- •2. Find five things in the texts to finish the sentence: “It reminds me of…”
- •2. Read the flowcharts given in the figure 1 and 2.
- •3. Read some information about creation of the flow charts in the Appendix 4-6 and create your own describing any experiment you made in the laboratory.
- •4. Create a list of rules related to the theme of the text given in the exercise 1. Share and compare the rules with your partners and think how they might be improved, choose the best ones.
- •5. Render the text given in the exercise 1.
- •2. Listen to two ways of giving presentations and see if you can hear some of the phrases above.
- •3. Read some advices on delivering effective presentations in the Appendix 7 and write your own presentation for the topic “Molecular symmetry”.
- •You ordered: Beckman du64 uv/VisSpectrophotometer
- •Unit 4 stereochemistry of reactions
- •Chiral Drug
- •1.Presentation: questions.
- •Unit 5 resolution of enantiomers
- •Resolution of enantiomers
- •1. Method of resolution is the title of the text in this section. What is the likely content of the article? Predict the methods which might be described.
- •3. Mark and talk about five things from the text you are glad to find out about. Talk in pairs about these things and why you chose them.
- •5.Render the text.
- •4. Think of three reasons you liked the text and three reasons you didn’t like it. Share and compare your reasons with other students. Find out how many other students share your opinion.
- •1.Presentation: useful tips.
- •3.Complete the sentence with the correct phrase.
- •Principles of Stereochemistry
- •Enantiomeric Relationships
- •Diastereomeric Relationships
- •Methods of determining configuration
- •The Cause of Optical Activity
- •Molecules With More Than One Chiral (Stereogenic) Center
- •Asymmetric Synthesis
- •Business english
- •Formal letter
- •1.Titles and addresses
- •2Covering the issues
- •3 Beginning your letter
- •4 Ordering ideas
- •5 Range
- •6 Ending the letter
- •Sample formal letter
- •Informal letter or email
- •1 Titles and addresses
- •2 Openings
- •3 Covering all the issues
- •4 Using informal language
- •5 Range
- •6 Connectors
- •7 Closing statements
- •Writing a tactful advice letter
- •How to write a request letter
- •Complaint letter
- •If necessary, add any further information:
- •Writing claim letter
- •Inquiry letter
- •Establish Your Objective
- •Determine Your Scope
- •Organize Your Letter
- •Draft Your Letter
- •Close Your Letter
- •Review and Revise Your Inquiry Letter
- •Sample Inquiry Letter __________Better Widget Makers, Inc.__________
- •5555 Widget Avenue
- •Appendices appendix 1 exclamations
- •Appendix 2 general conversation gambits
- •Appendix 3 the scheme of rendering the text
- •Appendix 4 flow charts
- •Appendix 5 graph
- •Appendix 6 reading and interpreting graphs
- •Types of Graphs
- •Appendix 7 presentations
- •Typescripts
- •Bbc Learning English. Talking Business
- •(Bbclearningenglish. Com)
- •Bibliography 1
- •Bibliography 2
2. Find five things in the texts to finish the sentence: “It reminds me of…”
3. How do these texts differ? What do they have in common?
4. Tell what was said in the texts about:
chiral, achiral, left hand, toothbrush, orbitals, molecular symmetry, symmetry operations, three-dimensional models,spectroscopy,valence theory,free molecule
5. Choose one of the three texts and render it.
Section B
1. Read the text below and decide which word or words combination best fit each gap. There are two words you don’t need to use.
Achiral, stereotopic, enantiomer, homotopic groups, enantiomers, chiral, enantiotopic groups, identical molecules, enantiotopic, constitutional isomers and diastereomers, constitutionally heterotopic and diastereotopic groups, symmetry.
STEREOISOMERISM OF MOLECULES
The stereomeric relationship between pairs of substances may be derived through the sequence of questions and answers represented by the flow diagram in Figure 1. In terms of properties, three broad categorizations arise:
1. a)_________________ Not distinguishable under any conditions, chiral or achiral.
2. b)________________The same in all scalar properties and distinguishable only under chiral conditions. Only molecules of which the point groups are Cn (n≥1), Dn (n>1), T, O, or I are chiral and can exist in enantiomeric forms.
3. c)_______________ Differ in all scalar properties and are distinguishable in principle under any conditions, chiral or achiral. Geometric isomers, which are related by the orientation of groups around a double bond, are a special case of diastereomers.
Molecules are d)__________if their molecular point groups do not include any Sn (n≥1) symmetry elements. Otherwise they are e)_________. An achiral molecule is not distinguishable from its own mirror image. This is often phrased as ``an achiral molecule is superimposable on its own mirror image.'' A chiral molecule is not superimposable on its mirror image. A molecule which is identical to the mirror image of another molecule is the f) __________of that molecule. According to the definitions above, an object is either chiral or it is not, it belongs to a particular point group or it does not. However, efforts have been made to define degrees of chirality and continuous measures of symmetry. The concepts of chirality and isomerism may readily be extended to pairs or larger assemblages of molecules, hence the reference to chiral and achiral environments above. Groups may be compared by internal comparison (groups in the same molecule) or by external comparison (groups in different molecules). One can also compare faces of a molecule in the same way as groups, since the comparison actually applies to environments. Thus, the two faces of the carbonyl groups of aldehydes, unsymmetrical ketones, esters, and other acid derivatives are enantiotopic. Reaction at the two faces by a chiral nucleophile will take place at different rates, resulting in asymmetric induction.
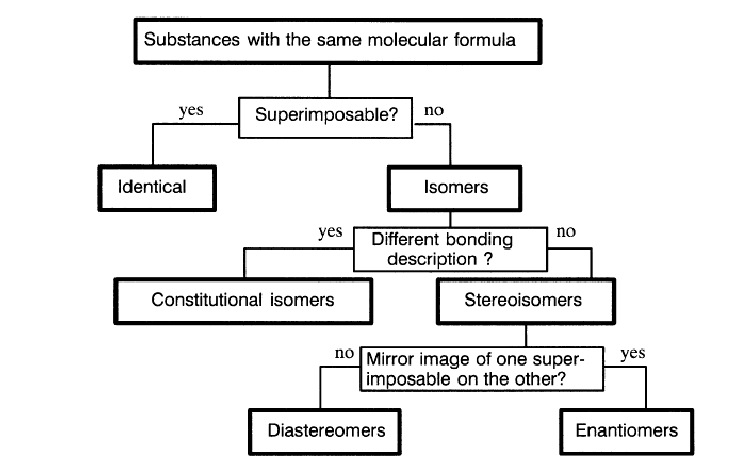
Figure 1. Flow chart for deciding stereomeric relationships between pairs of substances.
STEREOTOPIC RELATIONSHIPS OF GROUPS IN MOLECULES
Many of the ideas espoused in this and the next section are due to the work of Mislow. The concepts used to describe relationships between pairs of molecules may readily be extended also to pairs of groups within a molecule. This is particularly useful in determining the appearance of an NMR spectrum or the possibility of selective reaction at similar functional groups. Regions (such as faces of planar portions) around molecules may be similarly classified. The same relationships could also be applied to (groups of) atomic orbitals within the molecule. These are collectively referred to as “groups” for the purpose of the flow chart in Figure 2. From the analysis of Figure 1, three broad groupings of properties emerge:
1. g)_____________Not distinguishable under any conditions, chiral or achiral. To have homotopic groups, a molecule must have a finite axis of rotation. Thus the only molecules which cannot have homotopic groups are those whose point groups are C1;Cs;Ci , and C∞v.
2. k)_____________The same in all scalar properties, distinguishable only under
chiral conditions.
3. l)______________Differ in all scalar properties and are distinguishable under any conditions, chiral or achiral. Asymmetric molecules cannot contain homotopic or enantiotopic groups, only diastereotopic or constitutionally heterotopic groups. Groups may be compared by internal comparison (groups in the same molecule) or by external comparison (groups in different molecules).
One can also compare faces of a molecule in the same way as groups, since the comparison actually applies to environments. Thus, the two faces of the carbonyl groups of aldehydes, unsymmetrical ketones, esters, and other acid derivatives are m)____________. Reaction at the two faces by a chiral nucleophile will take place at different rates, resulting in asymmetric induction.
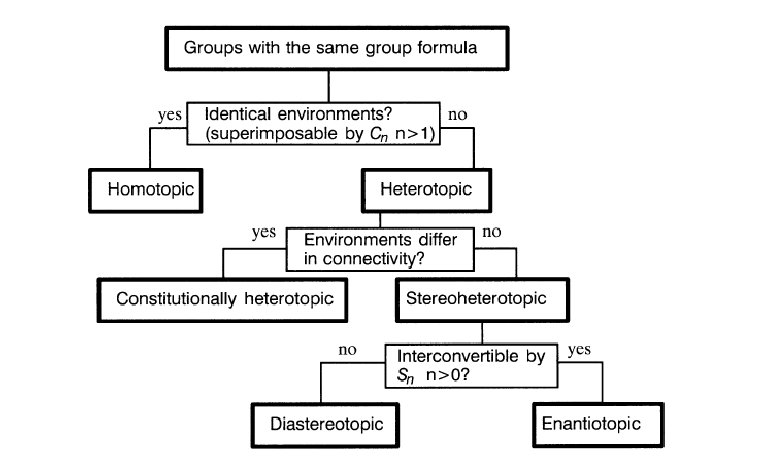
Figure 2. Flow chart for deciding stereotopic relationships between pairs of groups.
