
- •Contents
- •Foreword to the English translation
- •Preface
- •1 Introduction
- •1.1 Historical review
- •1.2 The birth of the concept of crystal growth
- •1.3 Morphology, perfection, and homogeneity
- •1.4 Complicated and complex systems
- •References
- •Suggested reading
- •2 Crystal forms
- •2.1 Morphology of crystals – the problems
- •References
- •Suggested reading
- •3 Crystal growth
- •3.1 Equilibrium thermodynamics versus kinetic thermodynamics
- •3.2 Driving force
- •3.3 Heat and mass transfer
- •3.4 Examples of mass transfer
- •3.6 Nucleation
- •3.7 Lattice defects
- •3.8 Interfaces
- •3.9 Spiral growth
- •3.10 Growth mechanism and morphology of crystals
- •3.11 Morphological instability
- •3.12 Driving force and morphology of crystals
- •3.13 Morphodroms
- •3.14 Element partitioning
- •3.15 Inclusions
- •References
- •Suggested reading
- •4 Factors determining the morphology of polyhedral crystals
- •4.1 Forms of polyhedral crystals
- •4.2 Structural form
- •4.3 Equilibrium form
- •4.4 Growth forms
- •4.4.1 Logical route for analysis
- •4.4.2 Anisotropy involved in the ambient phase
- •4.4.3 Whiskers
- •MAJOR FACTORS
- •METHODOLOGY
- •IMPURITIES
- •AMBIENT PHASES AND SOLVENT COMPONENTS
- •4.4.7 Factors controlling growth forms
- •References
- •Suggested reading
- •5 Surface microtopography of crystal faces
- •5.1 The three types of crystal faces
- •5.2 Methods of observation
- •5.3 Spiral steps
- •5.4 Circular and polygonal spirals
- •5.5 Interlaced patterns
- •5.6 Step separation
- •5.7 Formation of hollow cores
- •5.8 Composite spirals
- •5.9 Bunching
- •5.10 Etching
- •References
- •Suggested reading
- •6 Perfection and homogeneity of single crystals
- •6.1 Imperfections and inhomogeneities seen in single crystals
- •6.2 Formation of growth banding and growth sectors
- •6.3 Origin and spatial distribution of dislocations
- •References
- •7 Regular intergrowth of crystals
- •7.1 Regular intergrowth relations
- •7.2 Twinning
- •7.2.1 Types of twinning
- •7.2.2 Energetic considerations
- •7.2.4 Penetration twins and contact twins
- •7.2.5 Transformation twin
- •7.2.6 Secondary twins
- •7.3 Parallel growth and other intergrowth
- •7.4 Epitaxy
- •7.5 Exsolution, precipitation, and spinodal decomposition
- •References
- •Suggested reading
- •8 Forms and textures of polycrystalline aggregates
- •8.1 Geometrical selection
- •8.2 Formation of banding
- •8.3 Spherulites
- •8.4 Framboidal polycrystalline aggregation
- •References
- •Suggested reading
- •9 Diamond
- •9.1 Structure, properties, and use
- •9.2 Growth versus dissolution
- •9.3 Single crystals and polycrystals
- •9.4 Morphology of single crystals
- •9.4.1 Structural form
- •9.4.2 Characteristics of {111}, {110}, and {100} faces
- •9.4.3 Textures seen inside a single crystal
- •9.4.4 Different solvents (synthetic diamond)
- •9.4.5 Twins
- •9.4.6 Coated diamond and cuboid form
- •9.4.7 Origin of seed crystals
- •9.4.8 Type II crystals showing irregular forms
- •References
- •Suggested reading
- •10 Rock-crystal (quartz)
- •10.1 Silica minerals
- •10.2 Structural form
- •10.3 Growth forms
- •10.4 Striated faces
- •10.5 Growth forms of single crystals
- •10.5.1 Seed crystals and forms
- •10.5.2 Effect of impurities
- •10.5.3 Tapered crystals
- •10.6 Twins
- •10.6.1 Types of twins
- •10.6.2 Japanese twins
- •10.6.3 Brazil twins
- •10.7 Scepter quartz
- •10.8 Thin platy crystals and curved crystals
- •10.9 Agate
- •References
- •11 Pyrite and calcite
- •11.1 Pyrite
- •11.1.2 Characteristics of surface microtopographs
- •11.1.4 Polycrystalline aggregates
- •11.2 Calcite
- •11.2.1 Habitus
- •11.2.2 Surface microtopography
- •References
- •12 Minerals formed by vapor growth
- •12.1 Crystal growth in pegmatite
- •12.3 Hematite and phlogopite in druses of volcanic rocks
- •References
- •13 Crystals formed by metasomatism and metamorphism
- •13.1 Kaolin group minerals formed by hydrothermal replacement (metasomatism)
- •13.2 Trapiche emerald and trapiche ruby
- •13.3 Muscovite formed by regional metamorphism
- •References
- •14 Crystals formed through biological activity
- •14.1 Crystal growth in living bodies
- •14.2 Inorganic crystals formed as indispensable components in biological activity
- •14.2.1 Hydroxyapatite
- •14.2.2 Polymorphic minerals of CaCO3
- •14.2.3 Magnetite
- •14.3 Crystals formed through excretion processes
- •14.4 Crystals acting as possible reservoirs for necessary components
- •14.5 Crystals whose functions are still unknown
- •References
- •Appendixes
- •A.1 Setting of crystallographic axes
- •A.2 The fourteen Bravais lattices and seven crystal systems
- •A.3 Indexing of crystal faces and zones
- •A.4 Symmetry elements and their symbols
- •Materials index
- •Subject index

7.5 Exsolution, precipitation, and decomposition 145
Table 7.2 Regular intergrowth relations observed in minerals
Terms |
Examples |
|
|
Coaxial intergrowth |
amphibole and pyroxene (common c-axis) |
|
zircon and xenotime (common c-axis) |
Oriented overgrowth (epitaxial growth) |
rutile (100) on hematite (0001) |
|
chalcopyrite (112) on enargite (001) |
|
many other examples |
Symplektitic intergrowth (including |
quartz and feldspar |
celyphite rim, corona, reaction rim) |
plagioclase and magnetite |
|
garnet and quartz |
Mylmekite intergrowth |
quartz and plagioclase |
Micrographic intergrowth |
quartz and plagioclase |
Topotaxy (syntaxy) |
anatase, rutile |
|
pyrrhotite → marcasite pyrite |
|
|
|
|
This is an important relation in investigating the textures of crystal aggregates formed by physiological mechanisms.
7.5Exsolution, precipitation, and spinodal decomposition
In addition to epitaxial relations, characteristic textures appear due to the intergrowth of crystals of two different species in a certain crystallographic relation. Various terms have been used in the mineralogical field to describe textures, as summarized in Table 7.2 [15], [16]. Observations of descriptive and taxonomy type have been accumulated, since they show the origin of rocks and ores, but understanding the mechanism of their formation still remains a future subject of research.
These intergrowth relations are formed through the processes of crystal growth, phase transformation or decomposition associated with a decrease in temperature and pressure, or metasomatism due to the supply of new components from outside.
Coaxial intergrowth is a paragenetic relation that describes crystals of two different species growing with a common axis; the misfit ratios between the two crystals in the direction of the common axis are small, without exception. The formation of coaxial intergrowth can be understood to be one crystal conjunct to the other in an epitaxial relation, where both continue to grow. If a liquid of eutectic A–B component is solidified from one side (unidirectional solidification), crystals of the two phases A and B precipitate in dotted, columnar or lamellar (with common axis) form, and show unique textures for unidirectional solidification. This is a well known phenomenon in metallurgy.
146 Regular intergrowth of crystals
The solubility of a solid–solution component varies depending on temperature and pressure.
Even if crystals of a completely homogeneous single phase are formed at high temperature, a dissolved component is precipitated if the crystal experiences a decrease in temperature or an annealing condition. Many examples have been observed in metallurgy and mineralogy, and the terms “precipitation” and “exsolution” have been used in the respective fields. If aluminum alloy containing about 2% Cu (duralumin) is annealed, Cu precipitates in thin platy form parallel to a specific face of Al. Impurity nitrogen present in diamond in a substitutional form precipitates in the form of thin platelets of order 100 nm2 parallel to the {100} plane of the host diamond (known as type I diamond). Since the presence of these platelets disturbs the movement of dislocations, duralumin and type I diamond containing impurity components have higher plastic strengths than the purer Al metal and type II diamond, which contains less nitrogen.
Exsolution is the term used to describe crystal growth occurring in a solid phase; it proceeds through nucleation and growth processes. Dislocations or inclusions, around which strain is concentrated, are highly likely to be sites for heterogeneous nucleation, and also the host and the exsolved phases exhibit a specific crystallographic orientation relation so as to minimize the excess energy induced by the interface between them. In the above examples, the exsolved phase appears as thin platelets parallel to a low-index face of the host, but arrangements of needles, dots, and stars aligned in specific directions controlled by the crystal structure of the host phase are also often encountered. Ti substitutionally present in corundum exsolves as needle crystals of rutile, TiO2. In this case, exsolved TiO2 needle crystals assemble in three directions on the {0001} face controlled by the oxygen closepacked structure of the host phase. Since numerous reflected light beams are focused by the curved surface of cabochon-cut crystals, which act as a lens, reflected light “strings” appear at right angles to the needles. This is the origin of the “stars” in star ruby and sapphire. Such an effect is called asterism, and is observed in many gemstones.
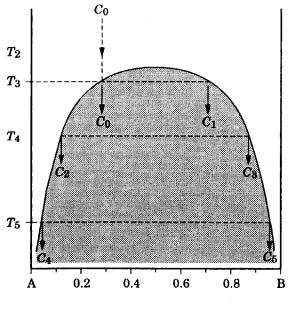
In the cases of silicate minerals, such as the feldspar group† and the pyroxene group,‡ the contents of the dissolved component are much higher than in the above cases. In such cases, even if (A, B) components completely dissolve to form a single solid–solution phase at high temperature, it is energetically favorable to be separated into two phases (A) and (B). Figure 7.14 is a phase diagram of a solid–solution of this type [17]. As a result, a texture consisting of alternating lamellae of two phases (A) and (B) is formed.
†Complex solid solution with KAlSi3O8 (Or), NaAlSi3O8 (Ab), and CaAl2Si2O8 (An) as the major components, where Or is orthoclase, Ab is albite, and An is anorthite.
‡Chain silicate minerals with MgSiO3 (En), FeSiO3 (Fs), and CaSiO3 (Wo) as the major components, where En is enstatite, Fs is ferrosilite, and Wo is wollastonite.
7.5 Exsolution, precipitation, and decomposition 147
temperature
composition XB
Figure 7.14. Phase diagram of a substance of (A, B) composition. In this system, one homogeneous solid–solution phase with composition C0 is formed at high temperature. As the temperature decreases, it is energetically more stable for (A, B) to be separated into two phases (A) and (B). A solid–solution with composition C0 will coexist as phases C0 and C1 at T3 in equilibrium, and, on lowering the temperature further, phases C4 C5 at T5 will be in equilibrium.
The widths of these lamellae depend on the thermal history. Many examples have been observed in minerals, and these lamellae widths are used to analyze the thermal histories.
When the thicknesses of the lamellae are of the order of the wavelength of visible light, an alternating multiple lamellar structure with different refractive indices is formed, which causes interference of light beams, giving rise to color. Bright interference color shown by laboradorite (Ab50An50 Ab30An70, where Ab NaAlSi3O8, An CaAl2Si2O8) is due to alternately exsolved lamellae of Ab and An components. Depending on the size or form of the exsolved lamellae, the structure can cause light scattering. Moonstones exhibiting what is known as the “moonstone effect” are such an example. In precipitation or exsolution, the formation of a new phase follows the form of crystal growth in a solid phase with a crystal structure, and involves nucleation and other growth processes that must overcome an energy barrier.
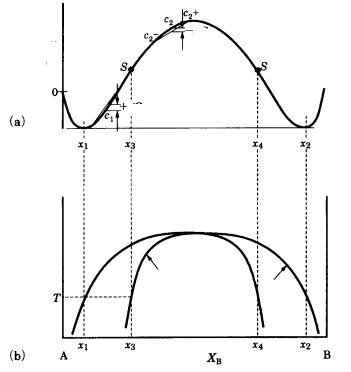
There are systems, however, in which the separation of a single phase into two phases as the temperature decreases is energetically favorable. Figure 7.15 indicates the phase diagram of such a phase [17].
148 Regular intergrowth of crystals
∆G2
mix ∆G
∆G1
temperature
spinodal |
solvus |
|
curve |
||
curve |
||
|
composition
Figure 7.15. Phase diagram of a phase showing spinodal decomposition. (a) In a regular solution having positive mixing enthalpy, Gmix has an opposite sign on the left and right sides of point S. So it is energetically favorable to have spinodal decomposition into x1, x3; x2, x4.
In this case, it is not necessary to overcome an energy barrier for nucleation to occur. Decomposition into two phases occurs without involving the process of nucleation. Separation into two phases in this type of system is called spinodal decomposition, and is seen among silicate minerals such as the pyroxene and feldspar groups. Generally, the boundaries between the two phases are wavy rather than flat, but the two phases are in a specific crystallographic relation.
Since the growth of a new phase is controlled by the structure of the host phase, the new phase should grow having a specific crystallographic relation with the host phase. This relation is applicable to the cases when a new phase, with composition of part of the host phase, is crystallized from the host phase by heating, or when a new phase is formed by the addition of another component. Since these processes involve the addition or departure of a part of the component, they are not the same as simple exsolution or spinodal decomposition. These phenomena are called syntaxy or topotaxy. This is because the new phase formation proceeds whilst keeping the basic structure of the host crystal, such as oxygen close-packing
