
- •Англійська мова
- •Смілянський технікум харчової промисловості
- •Передмова
- •For speciality 5.092503 (квп)
- •For specialities 5.080406 (соі), 5.080405 (прг), 5.091405 (окс)
- •For speciality 5.090227 (омр)
- •For speciality 5.090247 (мех)
- •For speciality 5.091715 (вцр)
- •For speciality 5.050202 (орг)
- •Тексти для читання, перекладу із словником та співбесіди Text 1
- •Evolution of the computer
- •Evolution of the computer
- •Further steps of evolution of the computer
- •Further steps of evolution of the computer
- •Electronic Computer Evolution
- •Electronic brain
- •Electronics - science of the future
- •Electronics
- •Temperature measurements
- •Text 10
- •Pressure measurements
- •Text 11
- •Electronic desk calculator
- •Specifications
- •Text 12
- •Standard platinum resistance thermometer ipts
- •Text 13
- •Model 1044k valve voltmeter
- •Text 14
- •Transistor receivers and energy transformers
- •Text 15
- •Transistor ultrasonic thickness gauge type 1103
- •Text 16
- •Eht stabilized power supply type 411
- •Specifications
- •Text 17
- •High - temperature glass electrode type эсп-31-06
- •Text 18
- •Germanium resistance thermometer tcг - 2
- •Text 19
- •The concept of electrical current
- •Text 20
- •Electrical measuring units and instruments
- •Для спеціальності квп text 16
- •Preferred value resistance box
- •Text 17
- •Dual transistor power supply type 423
- •Specification
- •Text 18
- •Auto-transformers
- •Text 19
- •Precision d.C. Amplifier.
- •Text 20
- •Protection and control equipment
- •Для спеціальностей соі, прг, окс text 16
- •Characteristics and usage of computers
- •Text 17
- •Computer elements
- •Text 18
- •Computer languages – high level and low level
- •Text 19
- •What is the microcomputer?
- •Text 20
- •What is the modem?
- •Для спеціальності омр text 16
- •Metal cutting processes and tools general description of lathes
- •Text 17
- •Machine-tools – a measure of man's progress
- •Text 18
- •Superhard materials from powder
- •Text 19
- •Lasers today and tomorrow
- •Text 20
- •Electro-ionizing laser (eil)
- •Text 17
- •Equipment for food industry. Universal mixers
- •Text 18
- •Equipment for food industry
- •Text 19
- •From the history of mechanics
- •Text 20
- •Machine elements screw fastenings
- •Для спеціальності вцр text 16
- •The science of chemistry
- •Text 17
- •Sorbitol powder with -crystallinity
- •Text 18
- •Citric acid by a gypsum-free process
- •Introduction
- •Fermentation
- •Text 19
- •Text 20
- •Sugars and non-sugar sweeteners
- •Для спеціальності орг text 16
- •Management
- •Text 17
- •How to win a market
- •Text 18
- •Contract and its features
- •Text 19
- •What is a manager
- •Text 20
- •Marketing today
- •Тексти для читання і переказу без словника Text 2 (1)
- •The Author of “Tom Sawyer”
- •Text 2 (2)
- •Academician I.V. Kurchatov
- •Text 2 (3)
- •Trying to Melt Wood
- •Text 2 (4)
- •Jack london
- •Text 2 (5) Read the text without a dictionary and retell it in English or in Ukrainian: Тhe Travels of Marco Polo
- •Text 2 (6)
- •Text 2 (7)
- •Cambridge
- •Text 2 (8)
- •Schools and sport
- •Text 2 (9)
- •The greatest american
- •Text 2 (10)
- •John lennon
- •Text 2 (11) Read the text without a dictionary and retell it in English or in Ukrainian: the cinema in britain
- •Text 2 (12)
- •Charles Darwin
- •Text 2 (13)
- •Maria curie
- •Text 2 (14) Read the text without a dictionary and retell it in English or in Ukrainian: yuri gagarin
- •Text 2 (15)
- •Mayflower
- •Text 2 (16)
- •Discovery of volta
- •Text 2 (17)
- •A. Conan doyle
- •Text 2 (18)
- •Text 2 (19)
- •Text2 (20) Read the text without a dictionary and retell it in English or in Ukrainian: famous british homes buckingham palace
- •Тематичні тексти для усного повідомлення (Topics)
- •1. About mу Family and myself
- •2. English
- •3. Our Technical Secondary School
- •4. My Future Profession
- •My Future Speciality (coi)
- •4. My Future Speciality (прг)
- •4. My Future Speciality (кіп)
- •4. My Future Speciality (омр)
- •5. Famous People of Ukraine
- •6. Ukraine
- •7. Kyiv
- •8. The United Kingdom of Great Britain and Northern Ireland
- •9. London
- •10. The Life of the Youth of Great Britain
- •11. The United States of America
- •12. Travelling by Air. Mr. Hawk Is Flying to Kiev
- •13. An educated man and computer
- •14. The Ties of Ukraine with England
- •15. Computer
- •16. Engineer and Technological Progress
- •17. Famous People of Great Britain
- •18. My Native Town
- •19. Technological Process of Sugar Production
- •20. Automation
- •Література:
Text 20
Read and translate the text with a dictionary, analyze the words and define the tense forms.
Machine elements screw fastenings
Machine parts are held together by parts: (a) working in tension, (b) working in shear, (c) creating friction, and (d) using both shear and friction forces.
Types of Fastenings. — All fastenings can be divided into two classes — disconnectable fastenings and permanent joints.
Disconnectable fastenings, in turn, are effected by: (a) bolts (Fig. 4 a, b) and screws, (b) wedges, (c) dowel pins (Fig. 4 c, d; Fig. 5 a), (d) keys.
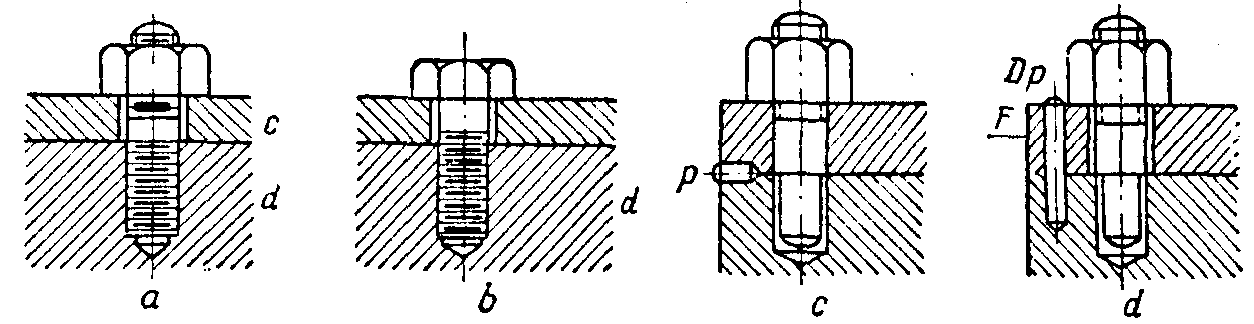
Permanent joints are obtained by means of: (a) press fits, (b) shrink fits, (c) rivets, (d) welding, brazing, and soldering and (e) casting.
Forms of Threads. — Screw fastenings are used for holding two or more machine parts together or for adjusting one part with relation to another. In screw fastenings the threads are made in several forms but are always of triangular-type single thread.
Screw threads are made right-hand and left-hand.
Answer the questions:
How are the machine parts held together?
What are the types of fastenings?
How are permanent joints obtained?
Для спеціальності вцр text 16
Read and translate the text with a dictionary, analyze the words and define the tense forms:
The science of chemistry
Chemistry is an experimental and theoretical study of the composition of matter and the changes that take place in matter. A chemical change involves changes in composition and in properties. A physical change involves only changes in properties with no change in composition.
Chemical changes are usually accompanied by the liberation or the absorption of energy in the form of light, heat or electricity.
All forms of matter consist of either pure substances or mixtures of two or more pure substances. Elements are the building blocks of matter. Compounds are combinations of elements. Most of the elements are metals and most of them will unite with other elements and form compounds. The formation of a compound from simpler substances is known as synthesis. Analysis is the process of breaking down a compound into simpler substances or its elements and thus is the determination of its composition. The composition of a pure substance never changes.
Every substance has physical and chemical properties. Physical properties include colour, smell, solubility, density, hardness and boiling and melting points. Chemical properties include the behavior with other materials.
Answer the questions:
What is chemistry?
What does chemistry study?
What does a chemical change involve?
What does a physical change involve?
What does all forms of matter consist of?
Text 17
Read and translate the text with a dictionary, analyze the words and define the tense forms.
Sorbitol powder with -crystallinity
General process description
The production of Y-crystalline Sorbitol powder is based on a melt crystallization process. The first step serves to remove the water almost completely. The resulting melt is then gradually cooled in a specially designed crystallizer whereby small Sorbitol agglomerates are formed. The coarse Sorbitol agglomerates are milled in a dry air atmosphere and finally screened to obtain the desired product fractions.
Evaporation
The Sorbitol solution (70 % w/w, assay 68 %) from the storage tanks is pumped via a feed tank to a thin film evaporator. It enters at the top of the evaporator through a distribution device from which the solution flows into the heated tube and runs downwards as a thin liquid film. To achieve a very low water content in the resulting melt, the whole process is carried out under vacuum.
Melt Crystallization
Sorbitol melt is continuously pumped via a pre-cooler, equipped with rotating scrubbers, to the crystallizer the Sorbitol melt is cooled by chilled water, which is circulating in the mixer and jacket. Cristal formation and growth start at a temperature of about 1000C. The transformation from the liquid to the solid form is an exothermic process which requires permanent removal of the crystallization enthalpy.
Answer the questions:
What is the production of -crystalline Sorbitol powder based on?
What does the first step serve to?
How is the whole process carried out, to achieve a very low water content?
