
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References

Protein Dephosphorylation and Protein Phosphorylation
with the upstream kinase MEK and the downstream transcription factor Elk. Thus, all these components of the MAPK pathway compete for the same binding site.97 It is not certain whether this mechanism of activation applies to all DS-MKPs.
Physiological role of the dual-specificity MAP kinase phosphatases
The archetypal dual-specificity phosphatase is the VH1 gene of vaccinia virus.5 A mammalian homologue of VH1 was discovered as a product of a growth factor-induced early response gene.98,99 Biochemical analysis revealed it to be a MAP kinase phosphatase (MKP-1).
Addition of serum to quiescent cells induces rapid but transient phosphorylation and activation of ERK2 (MAPK-1). This is sufficient to induce gene transcription and to promote entry into the G1 phase of the cell cycle.100 The activity of MKP-1 is expressed within 20 min and coincides with the dephosphorylation of the pTEpY motif in the activation segment followed
by the deactivation of the kinase. If synthesis of MKP-1 is prevented, or if its catalytic activity is suppressed by mutation, the duration of the activated state of ERK2 is greatly extended. Conversely, expression of a constitutively activated form of MKP-1 blocks G1 specific transcription and entry into S-phase.101,102 Thus MKP-1 acts as a negative regulator of ERK2, serving to attenuate the growth factor signal (Figure 21.19). In addition,
MKP-1 is a phosphorylation substrate of ERK2. When phosphorylated, it becomes less sensitive to ubiquitin-directed proteolysis and as a result, it accumulates.103
Despite all these promising results, this is certainly not the only pathway by which ERK2 is controlled because none of the mutations in MKP-1 predispose to tumour development. If this pathway were absolutely essential, then one might expect that a tumorigenic mutation would have emerged by now. Moreover, MKP-1-deficient mice appear to develop normally. Their fibroblasts respond normally with respect to the extent and timing of the expression of c-fos, indicating that the control of MAP kinase is unperturbed.105 We now know that MKP-1 is more active against members of the p38 and JNK kinases95 and that the ERKs are the preferred targets of MKP-3.106
Dual-specificity phosphatases in development
Most investigations of the dual-specificity MAP kinase phosphatases have focused on their roles in development. A particularly informative experiment, providing a direct demonstration of a physiological role for one of them in the regulation of MAP kinase activity, came from studies of dorsal closure during embryogenesis in Drosophila. In this process, lateral epithelial cells undergo cytoskeletal changes which enable them to spread out, providing
665

Signal Transduction
FIG 21.19 The role of MKP-3 in the regulation of receptor signalling. (a) All the phosphatases cause inactivation through dephosphorylation of one or both of the phosphoamino acid residues in the activation segment. Information from Farooq and Zhou.95 (2erk88). (b) Another example of phosphatases acting as a reset button. The scheme shows how dual specificity phosphatases operate in receptor signalling. Receptors activate the MAP kinase pathway, resulting in the activation of M3K (members of the ERK, JNK, or p38 subfamilies). A portion of these operate in the cytoplasm until they are dephosphorylated and inactivated by MKP-3. Others transfer to the nucleus to induce gene expression through phosphorylation of transcription factors. The product of one of the immediate early genes, MKP-1, enters the nucleus to inactivate M3K. MKP-1 can be induced by the synthetic glucocorticoid dexamethasone. Dephosphorylated and inactivated M3K accumulates in the cytoplasm were it is ‘recharged’ by occupied cell surface receptors. Information from Camps et al.104
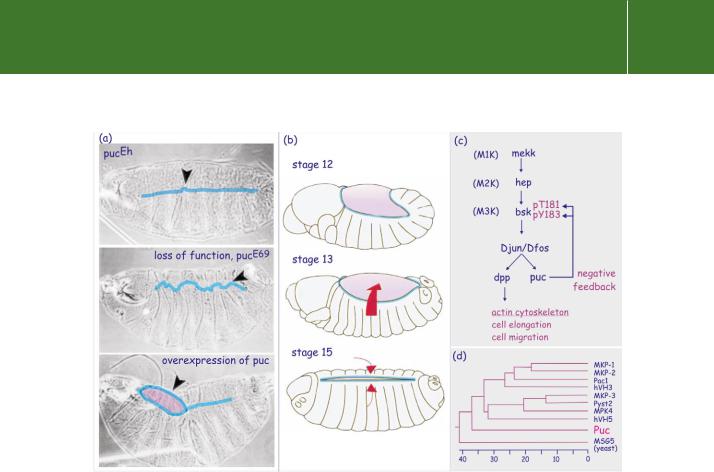
a cover for the dorsal region of the embryo107 (Figure 21.20b). The Drosophila homologue of the mammalian JNK, Bsk, induces the expression of puc, which encodes a dual-specificity phosphatase and negatively regulates activity of Bsk. The activity of Puc must be regulated precisely. Lack of Puc results in a disordered border between the dorsal epithelial cells (‘puckered’ phenotype) (Figure 21.20a). In excess it induces a phenotype characterized by patches of uncovered dorsal openings.108 Figure 21.20c shows the pathways that control dorsal closure.
The ERK-specific MKP-3 plays a similar role in the regulation of chicken limb outgrowth. This is initiated by FGF8 released from the epithelial cells
666
Protein Dephosphorylation and Protein Phosphorylation
FIG 21.20 Dorsal closure in Drosophila and the role of the dual specificity phosphatase puc. (a) Phenotypes of Drosophila embryos arising as a consequence of altered or enhanced expression of the puc gene. The weak mutant pucEh yields an almost perfect seal between the two sides of the ectoderm. Loss of function (pucE69) causes a puckered phenotype, in which the cells of the two borders penetrate each other’s territory. Over-expression prevents the leading edges reaching each other and the embryo reveals dorsal openings. (b) Stages in dorsal closure during Drosophila embryogenesis. The embryos are shown dorsal side up, with the anterior (future head region) to the left. At stage 12, a large part of the embryo is still covered by amnioserosa (pink). At stage 13, the ectoderm cell sheet extends towards the dorsal midline (red arrow). This movement takes about 2 h and is the consequence of the progressive flattening of cells which extend a leading edge towards the midline. At stage 15, the leading edges of both sides converge. The process of flattening and migration ceases. (c) The signal transduction pathway that controls dorsal closure. Positive control of flattening and migration occurs through induction of dpp (decapentaplegic, member of the TGF family of growth factors, see page 607) by a pathway involving mekk (M1K), hep (M2K), and bsk (M3K). When cells make contact, negative feedback, due to Puc-mediated dephosphorylation and inactivation of Bsk, arrests flattening and migration. (d) Phylogenetic tree analysis of puc, yeast MSG5, and human-dual specificity phosphatases.Image courtesy of Dr Martinez-Arias, Cambridge, UK. Embryonic stages according to Campos-Ortega and Hartenstein.109
that form the apical epidermal ridge. FGF8 causes proliferation of the underlying mesenchymal cells to form buds that extend into the limbs with differentiation of the mesenchymal cells into cartilage and then bone. MKP-3 is one of the genes up-regulated in mesenchymal cells by FGF8. Its inhibition of expression by siRNA, results in cell death in the mesenchyme.110 In this system, MKP-3 expression is regulated by the PI 3-kinase–PKB pathway.
667
