
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References

Signal Transduction
weakly expressed in non-confluent cultures of lung or foreskin fibroblasts, but is highly expressed in dense cultures.71 Later, genome analysis revealed that one of the mouse genes for susceptibility to colorectal cancer encodes a receptor tyrosine phosphatase, PTPRJ. Loss of heterozygosity of the human gene (DEP1) was noted in 19 out of 39 colorectal adenocarcinomas.72 DEP1 was the first tyrosine-specific PTP to be assigned a convincing role as a tumour suppressor relevant to the development of human cancers (the dual specificity phosphatase PTEN is also a tumour suppressor: see page 668). The receptor-PTK Met, which is up-regulated in several human tumours,
is a substrate of DEP1, so raising the possibility of a functional interaction between this phosphatase–kinase pairing in the progression of cancer.
CD45 and the regulation of immune cell function
The CD45 tyrosine phosphatase, expressed in all nucleated cells of haematopoietic origin, first alerted the attention of immunologists because of its great abundance (5–10% of membrane proteins). It is a transmembrane
tyrosine protein phosphatase having a receptor-like extracellular domain,73 but as with many other receptor-like PTPs, no specific activating ligand has been identified. Its importance became evident with the discovery that T lymphocytes lacking this antigen fail to become activated through the TCR, although they respond quite normally to stimulation through the IL-2 receptor.74–76 A total absence of surface CD45 causes severe combined immunodeficiency disease (SCID).77 Conversely, transgenic mice bearing an activating mutation in CD45 display lymphoproliferation, autoantibody production (and severe nephritis).78
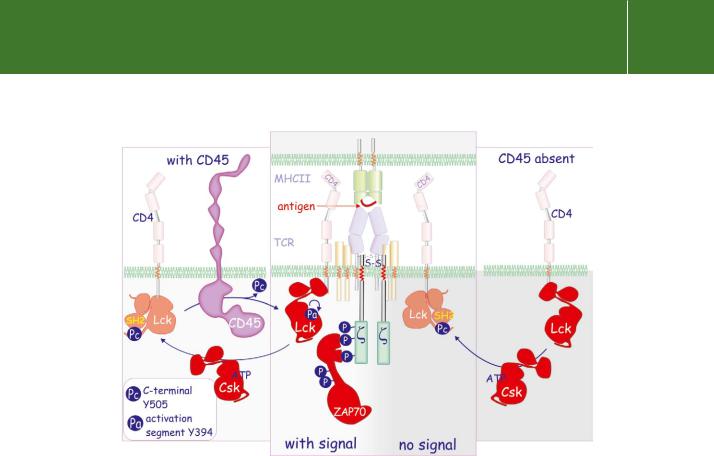
Normally, engagement of the TCR by an antigen-presenting MHC results in phosphorylation of the three ITAM motifs in the chains of CD3 (part of the TCR complex). This occurs through the intervention of the kinase Lck, bound to either CD4 or CD8 (Figure 21.14 and Figure 17.2, page 516; also see page 515). The fully phosphorylated chains then serve as docking sites for the formation of a signalling complex that transmits the signal into the cell. In cells lacking active CD45, Lck remains inactive and the subsequent cascade of tyrosine phosphorylations does not occur.
So where does the phosphatase activity of CD45 come into all this? Normally Lck activity is suppressed due to autoinhibition arising from the intramolecular interaction of its own SH2 domain with a phosphotyrosine residue situated in its C-terminus (“Pc” in Figure 21.14, the ‘closed conformation’). Lck is activated first through dephosphorylation by CD45 which exposes the kinase active site, and then through autophosphorylation in the activation segment.79 The fully active Lck phosphorylates the
ITAM motifs of the chains but the process only becomes efficient when engagement of the TCR by MHC peptides brings the catalytically competent Lck, bound to CD4 or CD8, into closer juxtaposition with the CD3 chains.
658
Protein Dephosphorylation and Protein Phosphorylation
FIG 21.14 Role of CD45 in T cell activation. TCR signalling is dependent on CD45. It maintains Lck in an open configuration by dephosphorylating the C- terminal tyrosine residue (Pc). Subsequent autophosphorylation in the activation segment (Pa) renders Lck catalytically competent. The kinase Csk does the opposite. It phosphorylates Lck at Pc which makes contact with the SH2 domain, causing it to assume a compact configuration. In the absence of CD45, TCR signalling is prevented. For details of the TCR, see Figure 17.2, page 516.
Similar mechanisms of activation through dephosphorylation apply in the action of Fyn (associated with the -chain of CD3) and Src.80 From this, it appears that CD45 maintains a pool of Lck, associated with CD4/CD8, in
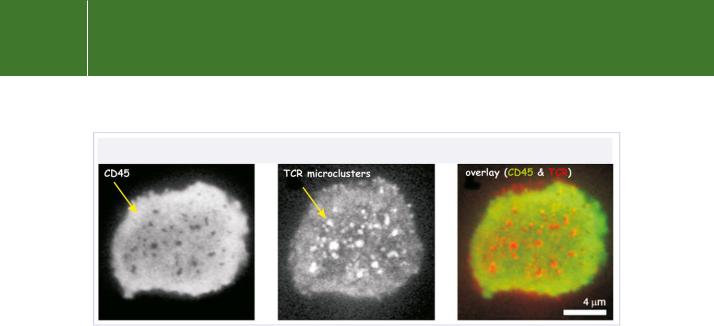
a competent state (but away from substrate). In this way, engagement of the MHC-peptide with the TCR leads to Lck-mediated phosphorylation of the CD3/TCRchains and thereby initiates TCR signal transduction.81 The localization of CD45 with respect to the major transmembrane signalling proteins in an immunological synapse has been visualized by laser confocal microscopy, and some images are shown in Figure 21.15.
In addition to Fyn and Lck, the Janus kinases (JAK) are also substrates of CD45.83 The JAKs are phosphorylated and activated following cytokine receptor ligation (IFN- , IL-3) and in turn phosphorylate the cytoplasmic tail of these receptors to create docking sites for proteins bearing an SH2
domain. Among these are the transcription factor STAT and Src family kinases. Tyrosine phosphorylation of STAT causes homoor heterodimerization
which induces translocation to the nucleus. Bone marrow-derived mast cells
659
Signal Transduction
FIG 21.15 Localization of CD45 and T cell receptors in the regulation of T cell activation. The fluorescent images are of T cells that have been placed on a lipid monolayer doped with MHCII antigen and ICAM-1. They form adhesive structures called microclusters. While CD45 is necessary for the supply of primed Lck (Figure 21.14), it is excluded from the TCR microclusters. This avoids down-regulation of the early tyrosine phosphorylation-based signalling events. The lack of overlap in the fluorescence signals from CD45 (green) and the TCRs (red) indicates their segregation. Only later (after ~20 minutes),
when so-called central supramolecular activation clusters are formed, is CD45 allowed to participate again and to terminate the activation process. From Varma et al.82
lacking CD45 respond more vigorously to IL-3 with respect to JAK2 activation, phosphorylation of STAT3 and 5 and proliferation.
Even now, the regulation of CD45 itself remains enigmatic. No ligand has been identified and structural analysis has failed to indicate how the phosphatase activity might be regulated
Regulating receptor PTPs
In direct contrast to the kinases, it appears that dimerization may the offswitch for the receptor tyrosine phosphatases. In order to test this idea, cells have been generated that express a chimaeric CD45 molecule that comprises its intracellular phosphatase-containing segment coupled to the extracellular domain of the EGF receptor. Application of EGF, expected to dimerize the receptor, is then found to switch off the phosphatase activity.84 By analogy to RPTP 85 and LAR (PTPRF), it was proposed that CD45 might form a symmetrical protein dimer in which the catalytic site of one component is blocked by a wedge structure formed by its partner (Figure 21.16). Separation of the two, exposing the catalytic site, would constitute the activation mechanism. In favour of this idea, the wedge region in CD45 is conserved
at the sequence level and ‘knock-in’ mice carrying a glutamate to arginine mutation in the wedge are subject to enhanced lymphoproliferation.
Against this however, a recent structural analysis of CD45 has provided no evidence of dimerization, nor did it reveal the presence of the proposed
660
