
- •Protein tyrosine phosphatases
- •Cytosolic PTPs
- •Transmembrane receptor-like PTPs
- •Tyrosine specificity and catalytic mechanism
- •PTPs in signal transduction
- •PTP1B, diabetes, and obesity
- •PTP1B as a possible therapeutic target for the treatment of type 2 diabetes and obesity
- •Redox regulation of PTP1B: reactive oxygen species as second messengers
- •Regulation of SHP-1 and -2
- •SHP-1, JAKs, and STAT5
- •SHP-2 and the Ras–MAP kinase pathway
- •Insight through the Noonan syndrome
- •Density enhanced PTP (DEP1)
- •CD45 and the regulation of immune cell function
- •Regulating receptor PTPs
- •Dual specificity phosphatases
- •Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
- •Physiological role of the dual-specificity MAP kinase phosphatases
- •Dual-specificity phosphatases in development
- •PTEN, a dual-specificity phosphatase for phosphatidyl inositol lipids
- •Serine/threonine phosphatases
- •Classification of the serine/threonine phosphatases
- •Regulation of PPPs
- •Phosphorylation of the catalytic subunits
- •Regulation by intramolecular domain interaction
- •Regulatory subunits of PP1
- •Inhibitors of PP1, PP2A, PP4, and PP5
- •PP1 in the regulation of glycogen metabolism
- •Regulation of glycogen metabolism: muscle
- •Regulation of glycogen metabolism: liver
- •PP2B (calcineurin)
- •Dephosphorylation of NFAT: immunophilins show the way
- •References

Protein Dephosphorylation and Protein Phosphorylation
FIG 21.16 Regulation of receptor tyrosine phosphatases. Dimerization is the turn-off signal. Inhibition is thought to occur through reciprocal interaction of the two wedge structures (in red) with the catalytic site (blue/yellow) which impedes substrate access. It is unclear whether or not ligands are involved in all this, and if they are it is unclear how they act to keep the receptors apart. (a) The structure of the D1 and D2 domains of LAR (PTPRF).
D1 is catalytically active, D2 is a pseudo phosphatase domain (inactive). Characteristic features of tyrosine phosphatases are indicated (Ptyr-loop, Q-loop, WPD-loop and the PTP-loop, which harbours the catalytic cysteine residue (in yellow). (b) Possible action of a hypothetical ligand of the LAR/RPTP phosphatases.(1lar86).
wedge. The catalytic site of D1 (membrane proximal catalytic domain, see page 644) appears to be unobstructed. This study did, however, indicate the importance of the catalytically inactive D2 domain (highly conserved within the tyrosine phosphatase family and across species). It concerns two flexible loop structures, acid and basic. Removal of the acidic loop diminishes CD3mediated activation of T cells. Moreover, phosphorylation of serine residues in the acidic loop by casein kinase 2 appears to play a role in CD45 function. From this we conclude that D2 serves an important purpose, though what it might be remains far from clear.
Dual specificity phosphatases
The dual-specificity phosphatases (DSP) form a subfamily of the tyrosine protein phosphatases. Under in vitro conditions, these enzymes catalyse the dephosphorylation of all three phosphoamino acids, pT, pS, and pY.
Examples include MKPs, VHR, KAP, CDC25, and PTEN. Although sharing no overall sequence similarity between themselves, nor with the tyrosine-specific phosphatases, the presence of the conserved PTP signature motif (I/V)HC-x5-R) indicates that they are members of this wider family. The catalytic domains
of VHR and KAP possess core structural features similar to the PTPs but appear to be truncated versions. Importantly, they lack the phosphotyrosinebinding loop and consequently the active site cleft has a depth sufficient
661
Signal Transduction
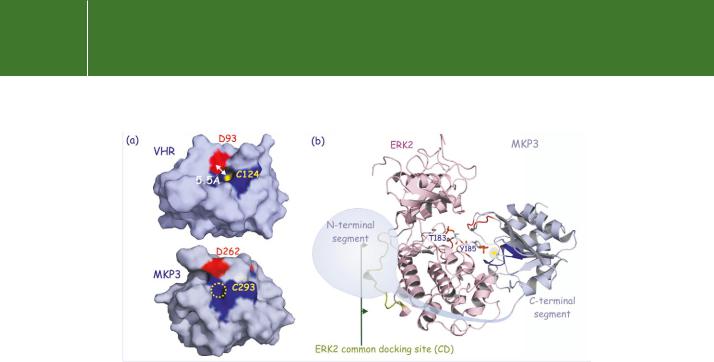
FIG 21.17 Structure of the dual specificity phosphatases VHR and MKP3. (a) Surface representation of VHR and MKP3. The PTP loop is dark blue and the aspartates D93 and D262, important for the dephosphorylation reaction, are red. The catalytic pocket measures 5.5 Å. The catalytic site cysteine in VHR is yellow. In MKP3 it is not exposed to the surface (no yellow spot visible). It appears that the binding of ERK alters the active site of MKP3 so as to expose the catalytic cysteine (C293). See also Figure 21.5. (b) Model illustrating the interaction of ERK2 with MKP3. The N-terminal segment of the phosphatase binds to the common docking site (CD) at the ‘back’ of ERK2 whereas the C-terminal segment binds to Y185 in the activation segment. The two segments are connected by a hinge region that folds around the C-lobe of the kinase. (1vhr,87 2erk,88 and 1mkp89).
to accommodate both pY and pT (Figure 21.17). Numerous dual specificity phosphatases have been characterized, they are subdivided in four different groups, each comprising various subfamilies (Figure 21.18).
Regulation of MAP kinases by dual-specificity protein phosphatases (DS-MKP)
Members of the MAP kinase family are phosphorylated in the TEY sequence by dual-specific kinases such as the MEKs (see page 334). They are dephosphorylated by the MAP kinase phosphatases (MKP), of which some are serine/threonine phosphatases (PP2A and PP2C) while others are tyrosine phosphatases (PCPTP1, STEP and HePTP).91,92 Yet others are DSPs (DS-MKPs).
Ascertaining the roles of the specific MKPs in vertebrates has been hindered by the great diversity of phosphatase enzymes that inactivate MAP kinases and also their functional redundancy. Rapid inactivation of ERK1 in PC12 cells reflects, in part, dephosphorylation of phosphothreonine,93 whereas in other systems, such as rat-1 fibroblasts, a dominant role for members of the PTP family is evident.94
To date, 13 human MKPs have been identified95 (Table 21.2). They are structurally and functionally distinct and are grouped into four categories.
662

Protein Dephosphorylation and Protein Phosphorylation
FIG 21.18 Domain architecture of the dual specificity phosphatases. Although characterized by the presence of a common signature motif, the DSPs are more diverse than the specific PTPs. As a consequence, numerous small subfamilies exist that possess a truncated version of the catalytic domain. Among other differences, this results in a shallower catalytic pocket. Both catalytically competent and pseudo phosphatases have been identified. Some accessory domains as well as interaction sites or post-translational modification sites are highlighted. Protein names and gene symbols do not always match. From Tonks.90
663

Signal Transduction
Table 21.2 MAP kinase phosphatases: Four categories of MAP kinase phosphatases are recognized. The first two comprise dual-specificity phosphatases of which members of the first group are directly induced by MAP kinases (immediate early genes) and are localized mainly in the nucleus. The second group are not induced by MAP kinases and act in the cytoplasm. The third category comprises members of the tyrosine phosphatase family (PTP) and the fourth comprises the S/T phosphatases PP2A and PP2C. Not all members of the MAP kinase family are equally susceptible as substrates; preferences are indicated for some.
Dual specificity phosphatases
MKP-1 (CL100) |
nuclear |
|
|
MKP-2 (hVH2) |
immediate early genes |
|
|
hVH3 (B23) |
growth factor or stress induced |
|
|
PAC1 |
growth factor or stress induced |
|
|
MKP-3 (Pyst1) |
cytosolic |
|
|
MKP-X (Pyst2) |
non-immediate early genes |
|
|
MKP-4 |
|
|
|
MKP-5 |
|
|
|
Tyrosine phosphatases |
|
|
|
PCPTP1 |
neuronal |
|
|
STEP |
targets ERK 1/2 & p38, not JNK |
|
|
HePTP |
lymphoid |
|
|
|
targets ERK 1/2 & p38, not JNK |
|
|
Serine/threonine phosphatases |
|
|
|
PP2A |
targets all MAP kinases |
|
|
PP2C |
p38 & JNK |
|
|
Dephosphorylation of both pY and pT is believed to occur by a similar mechanism as that described for PTP1B (page 647).
A remarkable degree of selectivity between substrate and phosphatase is achieved through the interaction of MKP-3 to a common docking domain (CD) adjacent to the catalytic domain on ERK2 (see Figure 21.17). Binding of MKP-3 then causes rearrangement and activation of the catalytic site.96 Importantly, catalytic activation of MKP-3 mirrors the selectivity of the docking domain, as the different members, JNK and p38, are neither able to bind nor to increase its catalytic activity. The docking site on ERK2 is also its point of interaction
664
