
- •Chemotherapy
- •Cytotoxic antibiotics and antimetabolites
- •The purine pathway to chemotherapy
- •Good drugs and bad
- •Combination chemotherapy
- •Alternative targets for cancer therapy: towards a scientific rationale
- •Inhibiting the EGF family of receptor kinases
- •The antibody approach: trastuzumab
- •The tyrosine kinase inhibitor approach
- •Erlotinib
- •Gefitinib
- •Imatinib: chronic myeloid leukaemia and the Bcr-Abl fusion story
- •Development of imatinib, inhibitor of c-Abl
- •Why is treatment of CML successful?
- •Molecular mechanism of inhibition by imatinib
- •Resistance due to mutations at different sites in the Abl moiety of Bcr-Abl
- •Other signal transduction components targeted for therapeutic intervention
- •Towards a different approach in testing cancer drugs?
- •References

Chapter 23
Targeting Transduction
Pathways for Research and
Medical Intervention
The several thousand drugs commonly prescribed in the clinic only involve some 200 molecular entities. Of these, at least half are G-protein-coupled
receptors and a large proportion of these are the so-called drug receptors, those that interact with small molecules (adrenaline, acetylcholine, serotonin, histamine, etc.). The lack of viable drug targets can be ascribed in large part to the fact that until about 1980, little was known about most receptors and still less about what they do and how they work.
As we move from the small molecules through peptide (e.g. cytokines) and protein hormones, the challenge of designing appropriate ligands becomes ever more complex. And then there is the challenge of discovering reagents that are selectively agonistic or antagonistic. Protein–protein interactions generally occur over extended and, importantly, flexible surfaces, but it
has become apparent that only small areas within these large surfaces,
735
Signal Transduction
Medications typically have several names. While in the process of development, they are referred to by
a cryptic code. This is partly for reasons of commercial security, and partly because the molecules are often so chemically complex that their full chemical name is too unwieldy to use.
Subsequently they acquire a generic name, which
(to the knowledgeable) usually gives a clue to their function; and they are generally advertised and sold under a trade name, which may vary from one country to another as well as from one manufacturer to another.
For example, a drug used to treat certain types
of cancer is imatinib. That’s its recommended international nonproprietary name (rINN), which always starts with a lower-case letter. It
is currently marketed under the trade names Gleevec (in the USA) or Glivec (in Europe and Australia) – these names always start with a capital letter. Its systematic chemical name is 4-[(4-methylpiperazin- 1-yl)methyl]-N-[4- methyl-3-[(4-pyridin-3- ylpyrimidin-2-yl)amino] phenyl]benzamide,
and it was coded during development as CGP57148B or STI-571.
so-called hotspots, are critical. Targeting these hotspots by small molecules that antagonize protein–protein interactions may thus be feasible, either by binding at critical interfaces or to allosteric sites (that modify the structure of the critical interface).1
That such an approach can be successful is demonstrated by the therapeutic success of the fungal metabolites ciclosporin A and tacrolimus (FK506), widely used as immunosuppressants. Both of these act on inhibitory calcineurinbinding proteins (cyclophilin and FKBP12 respectively) and the catalytic subunit of calcineurin (phosphatase PP2B) (see pages 230 and 683). Other examples are the vinca alkaloids, vincristine and vinblastine, which bind - tubulin at the interface of the / -tubulin dimer and inhibit polymerization, or taxanes (paclitaxel, docetaxel), which bind -tubulin at the inner surface of the microtubule and cause stabilization.
The pharmaceutical industry has responded strongly to the current surge of interest in signal transduction mechanisms, especially in the area of cancer treatment. Here, the need for new drugs is self-evident and this selective approach offers the possibility of developing compounds that may have less severe adverse effects than their cytotoxic precursors.2
Numerous highly specific receptor and protein kinase inhibitors have now reached clinical trials. We discuss the development of trastuzumab (Herceptin), gefitinib (Iressa), and imatinib (Glivec/Gleevec) and their application in the treatment of breast cancer, non-small-cell lung cancer, and chronic myeloid leukaemia respectively. We also provide a brief outline of cancer chemotherapy, the rationale behind the choice of the novel signal transduction targets, and resistance to cancer treatment. The concluding message is that fundamental research remains essential in order to understand the success or failure of novel cancer treatments and thus to make advances in this field.
Chemotherapy
The term chemotherapy was coined by Paul Ehrlich at the end of the 19th century to define the treatment of disease by chemical compounds. His idea was that each disease could be treated with a chemical compound specific for that disease. It was the task of the pharmacologist to seek out such compounds by systematic testing of substances that offer therapeutic potential.
Ehrlich himself sought a compound that would cure syphilis and his compound ‘606’ (out of 900 tested) was the arsenical salvarsan (Latin salvare meaning save or cure). Arsenic binds to sulfydryl groups and one of the consequences of this is the inhibition of the mitochondrial production of ATP. This is probably the therapeutic site of action of salvarsan. By chance, the organism Treponema pallidum, the cause of syphilis, is more sensitive to this
736

Targeting Transduction Pathways for Research and Medical Intervention
arsenical than the human host is. Salvarsan remained the treatment of choice until 1939, when it was superseded by sulfapyrazine.
The term chemotherapy is now generally reserved for the treatment of cancer. The drugs that are current the mainstay of chemotherapy are based on findings and principles of action that date back to around 1917, at the time when armies started to deploy mustard gas as a means to waft enemy soldiers from their trenches. Apart from appalling blistering, the military medics also noticed that soldiers exposed to mustard gas had a greatly reduced white blood cell count, sometimes even losing their bone marrow (‘bone marrow aplasia’). Being aware that an excess white cell count equates to leukaemia, they reasoned that mustard gas (or similar compounds) might be effective in the treatment of blood cell cancers.
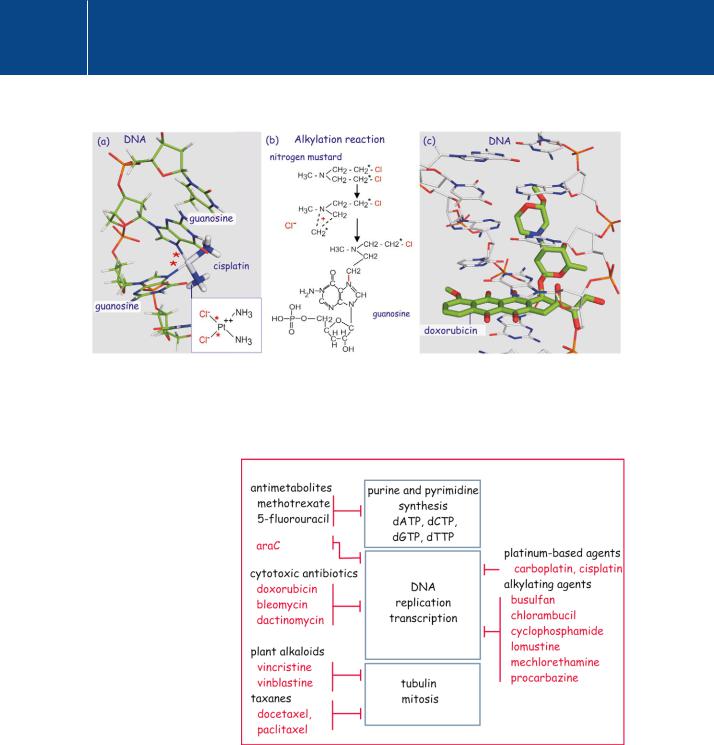
These compounds cause alkylation of guanine nucleotide bases in DNA, resulting in the inhibition of DNA-dependent DNA polymerase and arrest of cell division (see Figures 23.1 and 23.2). In order for cell division to proceed, the DNA has to be repaired (by excision and replacement of the affected bases). A whole range of compounds (cyclophosphamide, chlorambucil, lomustine, busulfan, etc.) have since been developed, all based on the same general principle.
The platinum-containing drugs cisplatin and carboplatin are variants on this theme (Figure 23.1).
The serendipitous discovery of cisplatin. In the early 1960s, a series of rather way-out experiments in the laboratory of Barnett Rosenberg yielded some peculiar results. An experiment designed to measure the effect of electric currents on cell growth yielded Escherichia coli that were 300 times the normal length. The effect was not due to the electrical fields themselves but to a chemical agent that was formed in a reaction between the supposedly inert platinum electrodes and components of the solution. The chemical agent was later identified as cisplatin. Further tests revealed that it prevents cell division but not other growth processes in the bacteria, which continue to elongate. When tested in mice it was found to be effective in eliminating tumours.3
Cytotoxic antibiotics and antimetabolites
The finding that metabolites of microorganisms can have cytotoxic effects gave rise to series of anticancer drugs. These compounds, such as the Streptomyces-derived doxorubucin, bleomycin, and dactinomycin, emerged in searches for potential antibiotics and are therefore sometimes referred to as cytotoxic antibiotics. They intercalate in the minor groove of DNA and have a number of effects that include chain fragmentation, hindering replication or transcription, or inhibition of topoisomerases (which catalyse the unwinding of DNA during chain replication). More designed are the metabolic inhibitors,
Mustard gas
(sulfur mustard) is not related to mustard in any way. It got its name because the impure gas has a yellow-brown colour and its odour resembles mustard and
garlic. Mustard gas is best described as a thioether having two reactive
Cl atoms. The loss of these in the aqueous environment makes it an electrophilic agent that can form covalent bonds with bases such as the guanine residues (N7) in DNA.
737
Signal Transduction
Fig 23.1 Mode of action of alkylating agents and cisplatin.
(a) Binding of cisplatin to two guanine residues in a DNA strand. Binding occurs at the same nitrogen that is bound by the alkylating agents. (b) Nitrogen mustard links to guanosine through methylene groups that have lost a chlorine atom. Two linkages are formed which are either intraor inter-strand. (c) Doxorubicin, a cytotoxic antibiotic, intercalates between the bases of two DNA strands. In doing so it prevents the action of DNA polymerase (1au5,4 2deS5).
Fig 23.2 Targets of some of the drugs used in chemotherapy.
Three main processes are targeted by the classic chemotherapy drugs. Most affect the making of DNA, both at the level of nucleotide synthesis (antimetabolites) and at the level of the polymerases (cytotoxic antibiotics, platinum-based drugs, and alkylating agents). The third target is the cell division machinery, inhibited by plant alkaloids and taxanes.
738
