
- •Adenylyl cyclase
- •Cyclic AMP: the first second messenger
- •cAMP is formed from ATP
- •Adenylyl cyclase and its regulation
- •Structural organization of adenylyl cyclases
- •Regulation of adenylyl cyclase
- •Regulation by GTP binding proteins
- •Regulation by phosphorylation
- •Aluminium fluoride
- •Forskolin
- •Cholera and pertussis toxins and ADP ribosylation
- •ADP-ribosylation and deribosylation: a general mechanism of cell control
- •Phospholipase C
- •First hints of a signalling role for inositol phospholipids
- •The phospholipase family
- •Phospholipase C
- •The isoenzymes of PLC
- •Regulation of PLC
- •References

Chapter 5
Effector enzymes coupled to GTP binding proteins: adenylyl cyclase and phospholipase C
Adenylyl cyclase
Cyclic AMP: the first second messenger
Looking back to the time when most wisdom in mammalian biochemistry was derived from experiments using rat liver (perfusions, slices,
homogenates, etc.), one gets a sense of the happy circumstance that led Sutherland to use dog liver in his investigations of hormonal activation of glycogenolysis1 (see page 34). During the growth and maturation of rats (6–60 days), the expression of -adrenergic receptors in the liver declines while that of the 1-adrenergic receptors increases.2 Adrenergic stimulation of glycogen breakdown in the liver of adult rats is therefore mediated primarily through the -receptors, which induce an elevation in the concentration of cytosol Ca2 (see Chapter 7). Using dog, Sutherland showed in 1957 that the
131
Signal Transduction
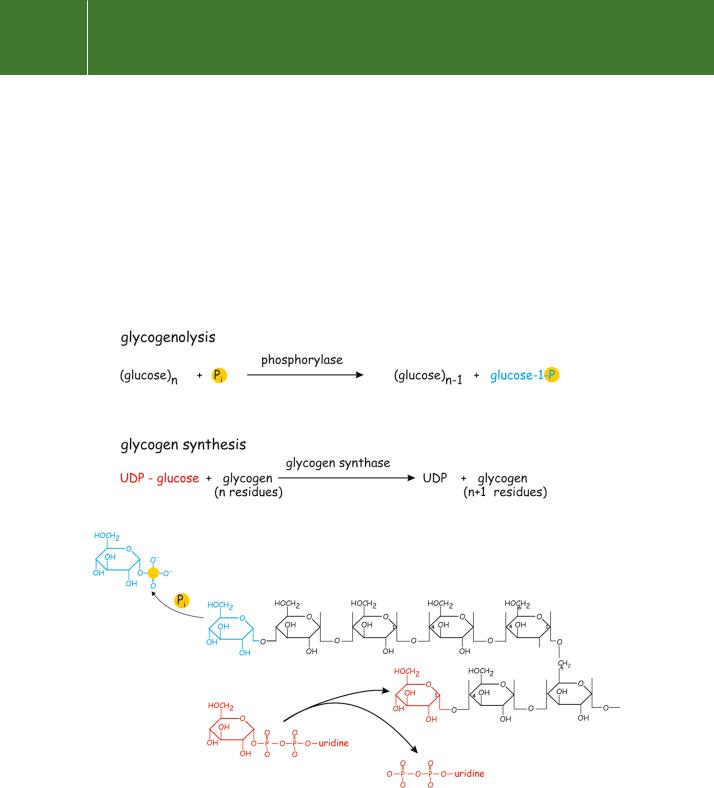
breakdown of glycogen, in response to adrenaline or glucagon, is consequent on the generation of a heat-stable and dialysable factor.1 This factor proved to be cyclic AMP, the first second messenger to be identified. The basic reactions of glycogen synthesis and breakdown are summarized in Figure 5.1.
Although cyclic AMP (henceforth cAMP) is most famously the intracellular signal for the breakdown of liver glycogen (and correspondingly for the shutdown of glycogen synthesis), it is a key mediator for many other important receptor responses. We return to these questions in Chapter 9. cAMP is also present in both the Eubacteria and Archaea, the other fundamental domains of life.3,4 Although its functions as a regulator in these organisms are very
Fig 5.1 The basic reactions of glycogen synthesis and breakdown.
Both the incoming (glycogen synthesis) and leaving (glycogenolysis) residues are attached to the non-reducing termini of the formed glycogen.
132
Effector enzymes coupled to GTP binding proteins
different from those in eukaryotes, its generation has signified a response to starvation throughout evolution. cAMP is synthesized in E. coli starved of glucose and it induces expression of sugar-metabolizing enzymes such as -galactosidase. In this case, it interacts with promoter elements in the
vicinity of the RNA polymerase site and is directly involved in the regulation of gene transcription. In yeast (unicellular, eukaryotic), cAMP is a growth signal. The presence of nutrients signals the activation of adenylyl cyclase through the RAS proteins. In starvation conditions, the ratio of GTPto GDP-bound RAS declines, either because of down-regulation of CDC25 (the guanine nucleotide exchange factor) or because of activation of IRA (GTPase activating protein). As a result, the cellular content of cAMP declines, growth ceases, and the cells switch to meiosis and spore formation. In the slime mould Dictyostelium discoides under conditions of starvation, cAMP is secreted by the individual cells as a signal for assemblage into a slug. Although it has generally been hard to discern functions for cAMP in plants,5 there is now good evidence that it acts as a regulator of ion channels for both K and Ca2 . The K channels in the guard cells of Vicia faba (broad bean) leaves appear
to be regulated by a cAMP-dependent kinase (protein kinase A, PKA6,7) and are indirectly sensitive to the actions of cholera and pertussis toxins that modulate the activity of GTP-binding proteins (see below).
cAMP is formed from ATP
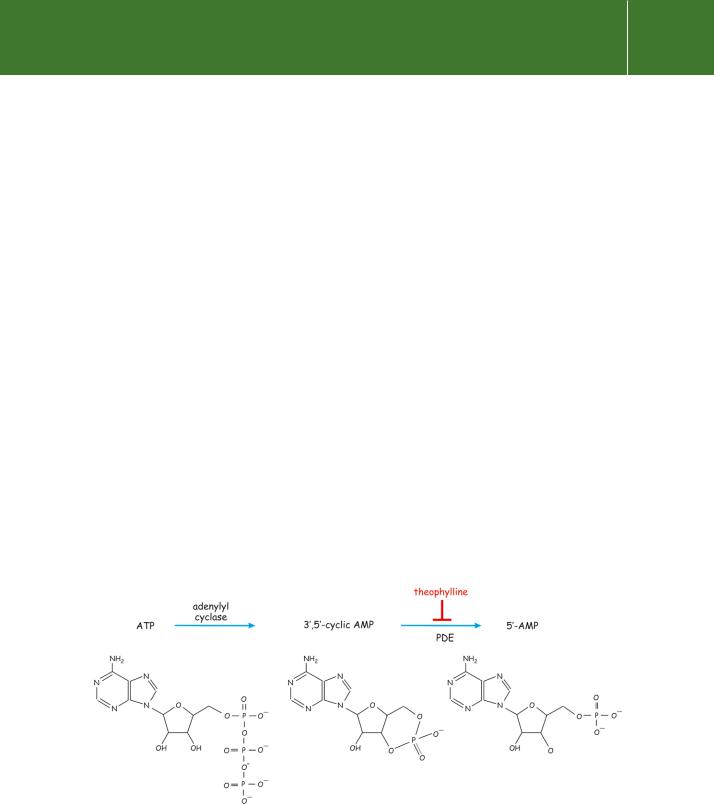
cAMP (strictly, 3 ,5 -cyclic adenosine monophosphate) is synthesized from ATP which is present in all cells at high concentration (5–10 mmol l 1). The formation of cAMP is catalysed by adenylyl cyclases and its removal, by conversion to 5 -AMP (adenylate), is catalysed by the action of phosphodiesterases (cAMP-PDE) (Figure 5.2).
Figure 5.2 Cyclic AMP is generated from ATP.
133

Signal Transduction
The preparation of adenyl cyclase in a simplified and purified form has been hampered by
the association with particulate material in the ‘nuclear’ fraction, by its lability and by its close association with the detergent Triton after solubilization. It is hoped that the enzyme may be solubilized by other procedures and
that more stable soluble preparations can be achieved.
Earl Sutherland, 1962
It is simple now to measure the level of cAMP with the use of kits, binding proteins, and specific antibodies. Not so simple for Sutherland and his colleagues who measured the product cAMP using a two-
stage assay procedure. The first involved incubation together with ATP, phosphorylase b, and fractions of liver homogenate (containing
phosphorylase kinase and PKA). This was followed up by measurement of the activity of the active product phosphorylase
a on its substrate glycogen.8,9
Adenylyl cyclase and its regulation
The enzyme
Adenylyl cyclase remained an activity without a proper molecular description for about 30 years, until 1990. At first, the possibility was entertained that more than one enzyme might be involved in the cyclization reaction. It was the importance of adenylyl cyclase in metabolic regulation and the simplicity of the reaction it catalyses, that provoked the intense investigation of its properties.
A number of proteins and other agents can activate or inhibit adenylyl cyclase. The known physiological activators and inhibitors include the subunits of the heterotrimeric G proteins. s and -subunits can activate, while i, o, and -subunits can inhibit. Also, Ca2 , through various mechanisms, can activate or inhibit. The plant product forskolin (described below) is a potent activator and this poses the interesting question of whether there might exist an endogenous counterpart in animal cells.
A major problem for research in this area was the fact that adenylyl cyclase, an integral membrane protein, generally comprises no more than 0.01–0.001% of total membrane protein. (An exception is the olfactory epithelium in which it comprises more than 0.1%.) The poor solubility of membrane proteins in aqueous media and their dependence upon the phospholipid environment for the maintenance of their tertiary structure makes them extremely difficult to isolate, purify and reconstitute into a form upon which sensible measurements can be made. Nevertheless the Gs-coupled form of adenylyl cyclase, present in many tissues, and a brain form of the enzyme have been purified.
From these preparations it became possible to obtain sequence information and thence a cDNA encoding the full length of adenylyl cyclase 1. The recombinant protein catalyses the conversion of ATP to cAMP and it can be activated by s.GTP and by Ca2 -calmodulin. Altogether, nine membranebound isoforms of mammalian adenylyl cyclase (AC1 to 9) have been cloned. (There is also a soluble isoform that is restricted to testis).
Structural organization of adenylyl cyclases
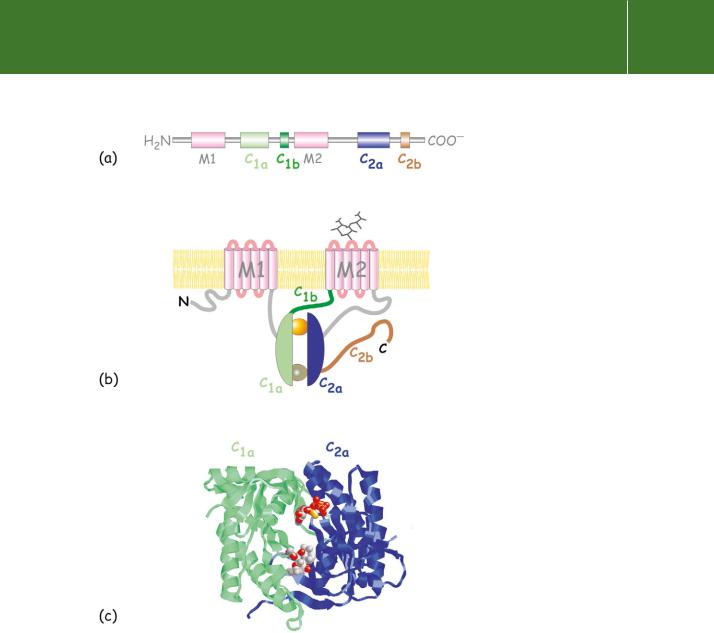
The structure reveals a number of distinct domains (Figure 5.3). Starting from the N-terminus, there is a predominantly hydrophobic domain, M1, organized as six membrane-spanning -helices linked on alternate sides of
the membrane by short hydrophilic loops. Next, there is an extensive (40 kDa) domain, C1, then another transmembrane domain M2, similar to M1, and finally at the C-terminus a second extensive cytoplasmic domain, C2 similar to C1.10 Beyond the general conservation of topology, the sequences of all forms of adenylyl cyclase are similar, having 40–60% identity overall. Within the C1 and C2 domains are the subdomains C1a and C2a that share 50% or even
134
Effector enzymes coupled to GTP binding proteins
Fig 5.3 Organization of adenylyl cyclases, depicting the main structural features of the mammalian adenylyl cyclases. (a) Domain architecture of adenylyl cyclase (b) General topology of the enzyme. With an even number of membrane spanning -helices, six each in the domains M1 and M2, the N- and C- termini are both in the cytosol. The catalytic unit comprises the two domains C1a and C2a. These contact each other to form binding sites for ATP, forskolin, and the - subunits of Gs and Gi.
(c) The molecular structure of the C1a and C2a dimeric cluster containing ATP S (upper spheres) and forskolin (lower spheres).
higher similarity. Residues from both C1 and C2 contribute to ATP-binding and catalysis.
Organized in head-to-tail fashion and having extensive contacts with each other, C1a and C2a comprise the catalytic centre of the enzyme.11 The interface between C1a and C2a accommodates two highly conserved nucleotide binding sites, only one of which appears to be crucial for catalysis. The other is the
site at which the activating substance forskolin binds. The binding site fors is distant (some 3 nm) from the catalytic site and has been localized to a small region at the N-terminus of C1a and to a larger region composed of a
negatively charged surface and a hydrophobic groove on C2a. The binding sites for the -subunits of Gi and Go do not compete with s. They too are formed
135
