
- •Adenylyl cyclase
- •Cyclic AMP: the first second messenger
- •cAMP is formed from ATP
- •Adenylyl cyclase and its regulation
- •Structural organization of adenylyl cyclases
- •Regulation of adenylyl cyclase
- •Regulation by GTP binding proteins
- •Regulation by phosphorylation
- •Aluminium fluoride
- •Forskolin
- •Cholera and pertussis toxins and ADP ribosylation
- •ADP-ribosylation and deribosylation: a general mechanism of cell control
- •Phospholipase C
- •First hints of a signalling role for inositol phospholipids
- •The phospholipase family
- •Phospholipase C
- •The isoenzymes of PLC
- •Regulation of PLC
- •References
Effector enzymes coupled to GTP binding proteins
Regulation of PLC
There are two ways in which PLC may be activated to hydrolyse PI(4,5)P2. One of these involves G proteins and the other a protein tyrosine kinase.
G-protein-coupled activation of PLC
The isoforms of PLC are activated by G-protein-mediated mechanisms
involving members of the Gq/11, Gi, or Go families. For proteins of the Gq/11 family it is the -subunit that conveys the activation signal, interacting at
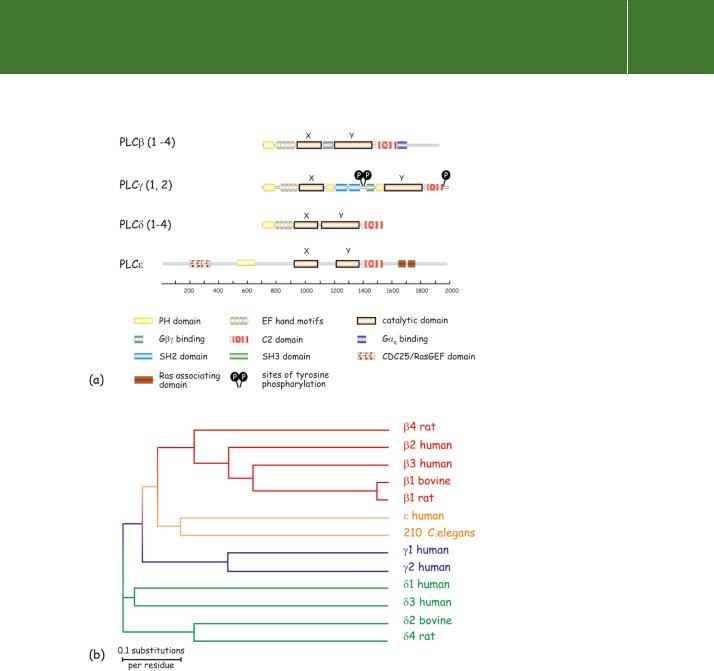
Fig 5.11 Organization of domains in the amino acid sequences of the PLC enzymes. (a) The main structural domains are indicated. In all of the inositide-specific PLCs, the catalytic domain is divided into two parts, designated X and Y. PLC has a minimalist design, having only the PH, C2, and EF-hand domains. The long ( 500 amino acids) C-terminal extension on PLC enables its association with membranes and
allows for regulation by -subunits of
the Gq/11 family of G proteins. In PLC the X and Y components are separated
by an extended stretch of more than 500 amino acids which includes two SH2 and one SH3 domain.
These mediate the interaction of the PLC enzymes with phosphorylated growth factor receptors and other signalling molecules. The sites of phosphorylation by receptor tyrosine kinases are indicated. Phospholipase C contains a RasGEF domain (see Chapter 4) at the N-terminus and two Ras associating (RA) domains at the C-terminus. (b) Evolutionary relationships of PI-specific phospholipases C based on sequence comparisons of the conserved X and Y catalytic domains. Adapted from Singer et al.51
151
Signal Transduction
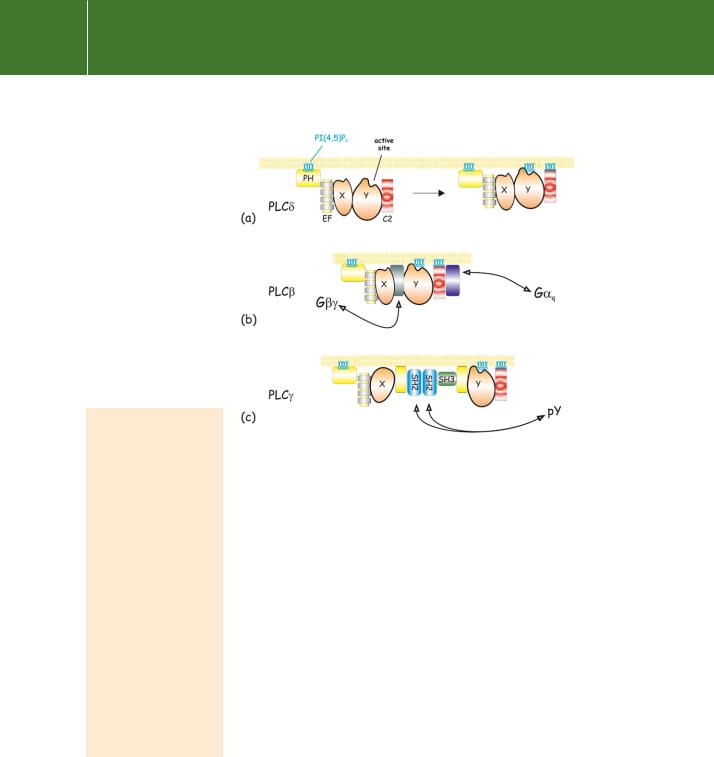
Fig 5.12 Membrane-association of PLC. (a) Initial attachment of PLC is through its PH domain. Subsequently, the C2 domain brings the catalytic site into contact with its substrate.
(b)PLC interacts additionally with G or G q to initiate catalysis.
(c)PLC also interacts with phosphotyrosines through its SH2 domains. The domains are colourcoded as in Figure 5.11. Adapted from Katan and Williams.54
Ligands that activate PLC through the - subunits of G proteins
of the Gq/11 family include 1-adrenergic
ligands, acetylcholine (M1 and M3), histamine, vasopressin, bradykinin, thromboxane A2, angiotensin II, bombesin, TSH, CXC chemokines, and endothelin.
Ligands that activate PLC 2 through subunits derived from Gi and Go include
1- and 2-adrenergic agonists, vasopressin (V2 receptor), acetylcholine (M2), formylmethionyl peptides, interleukin 8, and complement factor C5a.57
a site in the extended C-terminal region of these enzymes. In the case of receptors which communicate through Gi and Go it is the heterodimer that activates PLC 2 (and to a lesser extent PLC 1 and 3), interacting at a site between the X and Y catalytic subdomains.55 Deletion of the C-terminal section of PLC results in enzymes that fail to respond to q but still respond to subunits.56 In cells, this may be prevented by pertussis toxin which catalyses ADP-ribosylation of the -subunits of Gi and Go, but not the
members of the Gq/11 family. In addition, it is important to remember that the G proteins, their subunits, and the PLC effector enzymes are all membrane-
associated. Also that the substrate, PI(4,5)P2, and one of the products of reaction, DAG, are hydrophobic and are retained as integral components of the membrane phospholipid bilayer. In contrast, the other product, IP3, is water soluble and is released into the cytosol where it acts as a ligand to induce release of Ca2 from intracellular stores (see Chapter 7).
Regulation of PLC
PLC can be activated by -subunits of G12. Although relatively poorly characterized, G12 and G13 appear to be involved in the regulation of cytoskeletal changes and may themselves be activated by thrombin, lysophosphatidic acid, and some other mitogenic agents. A persistently activated mutant of G12 (GTPase-deficient Q229L) promotes cell growth and transformation characteristic of cancer cells (see Chapter 11). This phospholipase, which possesses a RasGEF domain and other
152
